How is "CO"_2 nonpolar?
1 Answer
Aug 14, 2017
By symmetry.
Polarity is a vector concept, i.e. a bond is polar because the electric dipole moment
vecmu = << mu_x, mu_y, mu_z >>
As vectors of identical magnitudes in exactly opposite directions add to cancel out completely, a "perfectly symmetrical" compound must be nonpolar, so that
sum_i vecmu_i = 0
For
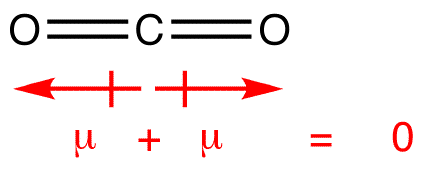 http://www.ochempal.org/
http://www.ochempal.org/
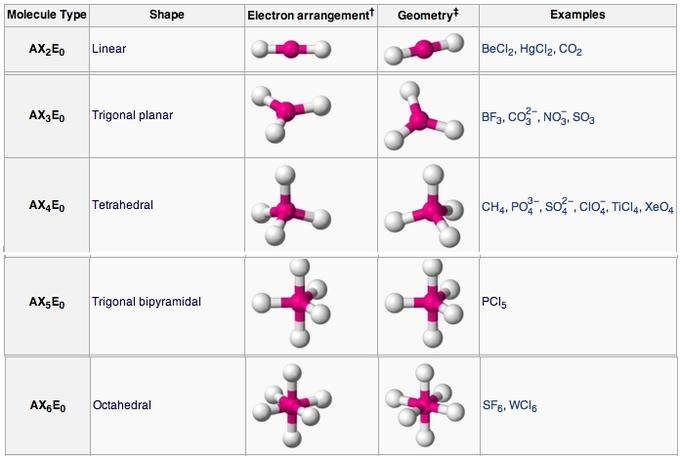
So, what do we mean by "perfectly symmetrical"? We mean the parent geometries of each so-called VSEPR structure, i.e. the ones with no lone pairs of electrons:
- two-atom linear, e.g.
"N"_2 - three-atom linear, e.g.
"CO"_2 - trigonal planar, e.g.
"BF"_3 - tetrahedral, e.g.
"CCl"_4 - trigonal bipyramidal, e.g.
"PF"_5 - octahedral, e.g.
"SF"_6 - etc.
all of which were NONPOLAR as listed above.
 https://figures.boundless-cdn.com/
https://figures.boundless-cdn.com/
The following, more usual examples, are POLAR:
"NO"^(+) , i.e.:"N"-=stackrel((+))("O": ) , two-atom linear"N"_2"O" , i.e.:stackrel((-))ddot"N"=stackrel((+))"N"=ddot"O": , three-atom linear"AlF"_2"Cl" , trigonal planar"CH"_3"Cl" , tetrahedral"PF"_3"Cl"_2 , trigonal bipyramidal"SF"_5"Cl" , octahedral

