Which direction do chloride ions flow when molten #"NaCl"# undergoes electrolysis?
2 Answers
Because the anode is always the site of oxidation; just as the cathode is always the site of reduction.
Explanation:
In electrochemistry, whether electrolysis or galvanic process, the electrodes are always defined in terms of the chemistry that takes place at the electrodes. The anode is the site of oxidation and the cathode is the site of reduction.
In the aqueous electrolysis of NaCl, if the concentration of the chloride ion is high, it will be the preferred oxidation at the anode. If the concentration of the chloride ion is low, water will be the preferred oxidation at the anode and oxygen will be observed from the electrolysis of water. When chloride oxidation dominates, the process is referred to as 'overvoltage' and is the foundation for the chlor-alkali membrane cell that produces gaseous molecular chlorine and concentrated NaOH solutions. By maintaining a high concentration of chloride ion in the system feed, chlorine gas is produced along with NaOH.
The electrolysis half-reactions for the chlor-alkali membrane cell are as follows:
Reduction Process at the Cathode:
Oxidation Process at the Anode:
Net Cell Reaction:
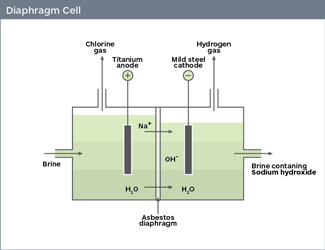
The anode is positively charged in an electrolytic cell (where electrolysis occurs), so it attracts the negatively charged chloride ions.
Explanation:
The chloride ions are oxidized at the anode, each losing one electron. The oxidized chlorine atoms then react to form chlorine gas,
#"2Cl"_((aq))^(-) -> "2Cl"_"2(g)" + "2e"^(-)"#
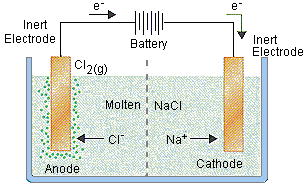
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch20/faraday.php



