Question #2f3a0
1 Answer
It's the other way around. The trimethyl compound is nonpolar. Here's why.
Explanation:
Shapes
Both molecules have five ligands about the

Orbitals
We say that the
However, the equatorial and axial orbitals form two different sets.
We can view the equatorial set as being formed from one
The axial set is formed from one
Bent's rule
Bent's rule states that atomic
The corollary is that orbitals with less
Structures
Hence, they will preferentially occupy the axial positions.
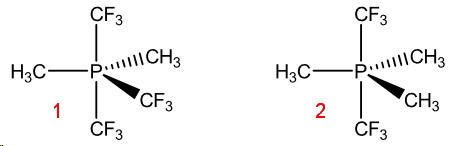
(Adapted from hope.simons-rock.edu)
In the trimethyl compound (2), the
In the dimethyl compound (1), one of the

