Question #31d2d
2 Answers
Explanation:
The first thing you need to do here is to use the molar mass of silicon to convert your sample from grams to moles.
Silicon has a molar mass of
#11.8 color(red)(cancel(color(black)("g"))) * "1 mole Si"/(28.0855color(red)(cancel(color(black)("g")))) = "0.42015 moles Si"#
Now, to convert the number of moles to number of atoms, use Avogadro's constant, which serves as the definition of a mole.
#color(blue)(ul(color(black)("1 mole Si" = 6.022 * 10^(23)color(white)(.)"atoms of Si"))) -># Avogadro's constant
So, in order to have
#0.42015 color(red)(cancel(color(black)("moles Si"))) * (6.022 * 10^(23)color(white)(.)"atoms Si")/(1color(red)(cancel(color(black)("mole Si")))) = color(darkgreen)(ul(color(black)(2.53 * 10^(23)color(white)(.)"atoms Si")))#
The answer is rounded to three sig figs, the number of sig figs you have for the mass of silicon.
Explanation:
There are several good Pinterest charts that show how to make molar conversions.
https://www.pinterest.com/pin/195484440057126887/
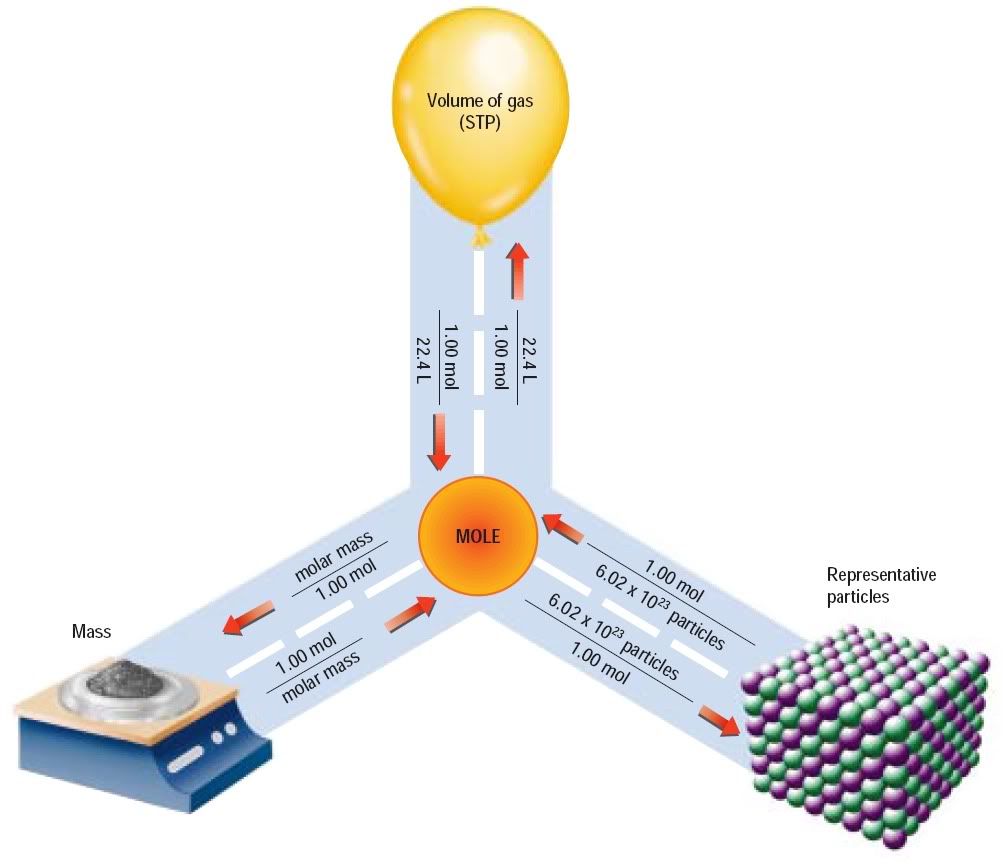
We have a mass, and we want the number of atoms of a specific element. So, we need to calculate the number of moles that mass represents in order to use Avogadro's number to find the number of atoms.
From the Periodic Table we find Si has a gram molecular weight of 28.1 g/mole. Therefore, the number of moles of Si here is 11.8g/28.1g/mol = 0.42 moles Si.
Multiply this by Avogadro's number of


