Question #8e3d7
1 Answer
3 atoms - 2 of which are hydrogen, and 1 is oxygen.
Explanation:
Let's take a look at the molecule of water.
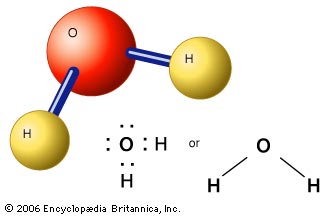
Here, we have 3 diagrams that indicate the molecule having 3 atoms: 2 of which are hydrogen atoms.
This makes sense, because water's chemical name is dihydrogen monoxide.
=> a molecular compound's naming system is based around the number of atoms each element has, and this corresponds to the prefix used. Di- = 2, mono- = 1.
A simple way to determine the number of atoms a chemical has, is to write out its chemical formula. Count all the different elements and its subscripts and add them out:
#H_"2"O# => 2 hydrogen, and 1 oxygen (no subscripts indicate 1).
Don't be confused when a multivalent element is reacting:
#Pb(SO_"4")_"2"# => sulfate by itself has 4 oxygen atoms and 1 sulfur atom. Multiply this by 2 (because of the subscript outside the bracket), and add another, due to lead's 1 atom.
The chemical name of this compound is lead (IV) sulfate. Notice how the subscripts were reduced, thus the number of atoms corresponded to that respectively.
Hope this helps :)

