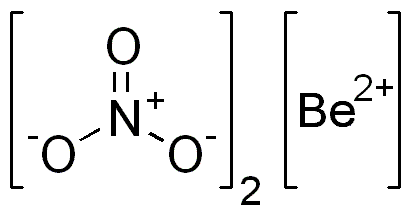Question #bd2d0
1 Answer
Explanation:
Start with the fact that beryllium,
In order to become stable, it donates these electrons to form
"Be"^(2+) -> the beryllium cation
The nitrate anion is actually a polyatomic ion that carries a
"NO"_3^(-) -> the nitrate anion
Since beryllium nitrate is a neutral compound, the overall positive charge coming from the cations must be balanced by the overall negative charge coming from the anions.
This means that you will need two nitrate anions in order to balance the
Therefore, you will have
["Be"]^(2+) + 2 xx ["NO"_ 3]^(-) -> "Be"("NO"_ 3)_ 2