Question #9f42f
1 Answer
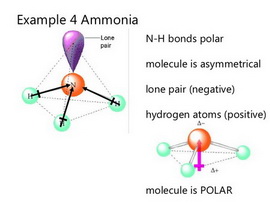
The three factors that cause the ammonia to be polar are the difference in electronegativity between hydrogen and nitrogen, the geometry of the molecule, and the presence of the lone pair.
Explanation:
There is a difference between the electronegativity of hydrogen and nitrogen. Nitrogen has an electronegativity of 3.0, while hydrogen has an electronegativity of 2.2. The electronegativity difference of 0.8 makes each of the three hydrogen-nitrogen bonds polar.
(From SlideShare)
The molecule has a trigonal pyramidal geometry. The nitrogen on the top of the pyramid has most of the negative charge. The three hydrogens on the bottom of the pyramid have the positive charge.
The nitrogen atom has a lone pair of electrons. These increase the negative charge on top of the nitrogen and add to the polarity of the molecule. We can see this in the electrostatic potential map of ammonia.
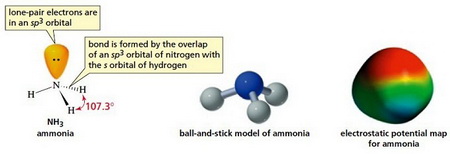
(From Kshitij IIT JEE)
So, the three factors that cause the molecule to be polar are the polar bonds, the molecular shape, and the presence of the lone pair.

