Question #d772b
2 Answers
I tried this:
Explanation:
You have that an electron in jumping from one level to another (as in your case, going down) will release energy in form of a photon of light.
The energy correspondent to this jump can be evaluated using a formula from Bohr's Theory (you can find it in the part of Quantum Physics of your book near Bohr's Postulates or Hidrogen Spectrum or at http://hyperphysics.phy-astr.gsu.edu/hbase/hyde.html):
We start using:
Where:
To get:
For semplicity I now change it into Joules to get the usual units for frequency and wavelength:
I know that:
so
I can get the frequency
so:
and for the wavelength
By the way
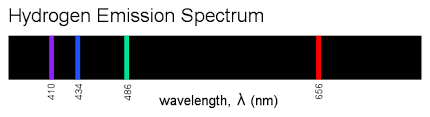
Explanation:
If you are working in electron volts then the energy of the electron in a particular energy level is given by:
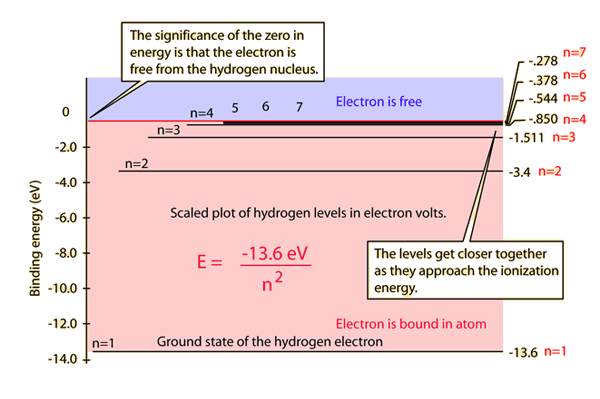
Where n is the principal quantum number.
When n = 2:
When n = 4:
This means the transition energy will be the difference between the 2 values:
The electron volt is not the standard unit for energy so we must convert it to Joules. To do this we multiply by the electronic charge:
To find the frequency f we use the Planck Expression:
Where h is the Planck Constant.
The photon will travel at the speed of light c . This is a universal constant:
The relationship we need is :


