What is the structure of an organic species that has a formula of #C_9H_18# in which ALL the hydrogens are equivalent?
2 Answers
Explanation:
I could not find a picture of this, and for all I know the molecule is unknown. A cyclopropyl ring, however, substituted with 6 methyl groups, fits your requirement. There are 6 methyl carbons attached to the three-membered ring, and all of them are
Anor277 is correct! hexamethylcyclopropane!
Explanation:
To solve this, one must first recognize that the only way to get all primary hydrogens is to have all the hydrogens on methyl groups,
There are 18 hydrogens.
That means there are 18/3 methyl groups.
That means we will need 6 carbons for the methyl groups.
That leaves 3 carbons to make up the support structure.
You could put them in a straight chain, but when you do that you end up with too many hydrogens and two of those hydrogens are tertiary:
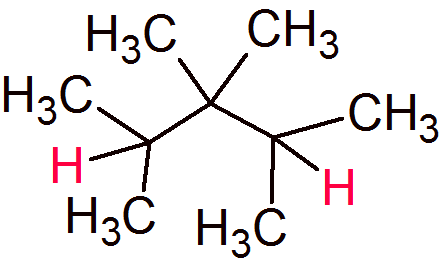
This is the same problem you have with alkanes: two too many hydrogens for a linear alkane. You solve that by making it a cycloalkane!
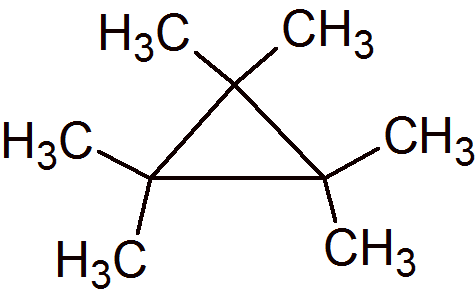
And there you have it! At least, that's how I reasoned through it.


