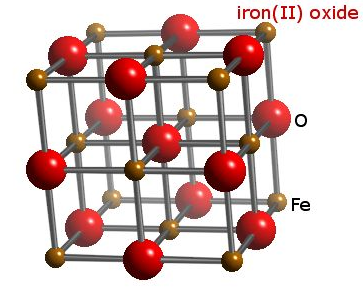
Question #d0643
1 Answer
Explanation:
The idea here is that ideally, iron(II) oxide is made up of iron(II) cations,
However, it is quite common to have cases where some of the iron(II) cations are missing from the lattice structure
In order for the overall positive charge to balance the overall negative charge, i.e. in order to continue to have a neutral compound, some of the remaining iron(II) cations are oxidized to iron(III) cations,
More specifically, for every iron(II) cation that is missing from the lattice structure, two iron(II) cations are being oxidized to iron(III) cations.
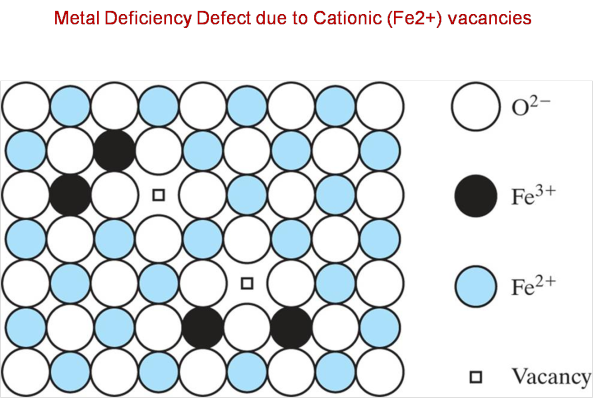
Now,
Since it's easier to work with whole numbers, you can say that for every
In other words, for every
Since you know that for every missing iron(II) cation, two iron(II) cations must be oxidized to two iron(III) cations in order for the positive charge of the cations to continue to balance the negative charge of the anions, you can say that this lattice structure will contain
#overbrace(2 xx "7 Fe"^(2+))^(color(blue)("2 for every missing Fe"^(2+))) = "14 Fe"^(3+)#
This means that out of every
#"14 Fe"^(3+)# #"cations"# #93 - 14 = "79 Fe"^(2+)# #"cations"#
Therefore, the fraction of iron(II) cations that you get for every
#(79 color(red)(cancel(color(black)("Fe"^(2+)color(white)(.)"cations"))))/(93 color(red)(cancel(color(black)("cations")))) = 79/93#