Question #04f3c
1 Answer
Formaldehyde is a naturally occurring organic compound with the formula CH2O
a)
As the carbonyl groups in aldehyde are polar because of the difference of electronegativity of the carbonyl carbon atom and oxygen they are both prone to electrophilic attacks at the oxygen atom and nucleophilic attacks at the carbonyl carbon atom.
The carbon acts as an electrophilic centre and the
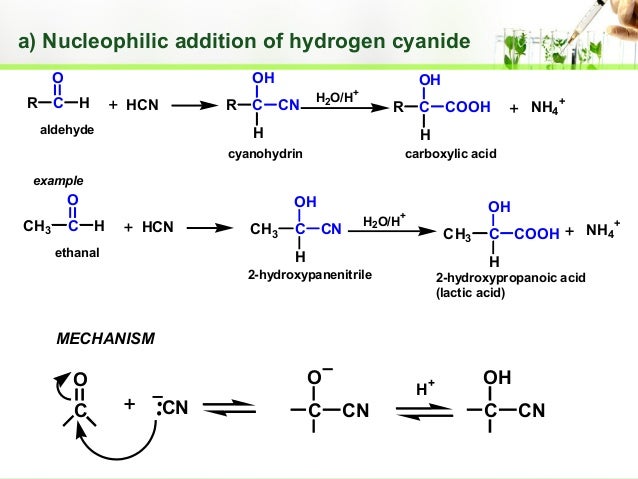
b)
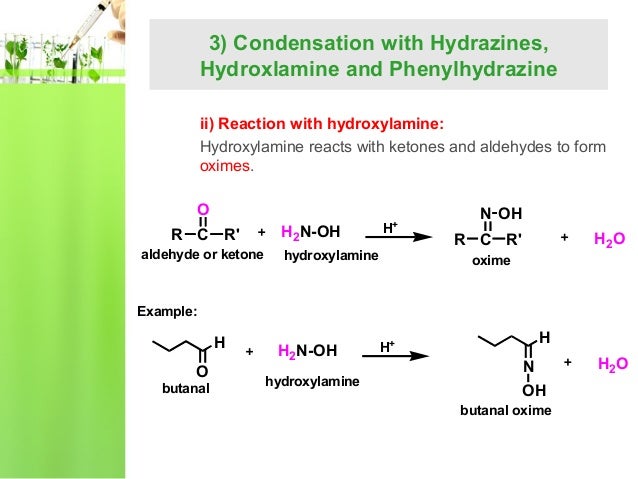
This is an electrophilic attack by 2 protons on a oxygen ion and this forms water which is good leaving group and so leaves the aldehyde. The
This is how
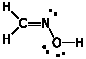
c) C2H5OH
Ethanol does not react with HCN. Did you mean "ethanal"?That's a different thing

