Why does the ionization energy of the halogen atoms DECREASE down the Group?
1 Answer
Apr 23, 2017
Would you not expect this order of ionization energies?
Explanation:
Ionization energy interrogates the process:
Now ionization energies increase across a Period (from left to right as we face the Table), but decrease DOWN a Group. As a new valence shell is occupied down the Group, the more distant valence electrons should be LESS tightly held by the nuclear charge, even tho
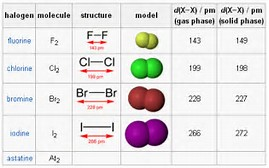 lookfordiagnosis.com
lookfordiagnosis.com
I can just read the diagram with glasses. I hope your eyes do better.

