Why is morphine a weaker base than piperidine?
1 Answer
I suspect the reason involves steric hindrance.
Explanation:
The structure of piperidine is

Piperidine is a weak base with
It has a cyclohexane ring structure in which the lone pair on the nitrogen atom is quite accessible to an attacking acid.
We can see this in both the ball-and-stick model
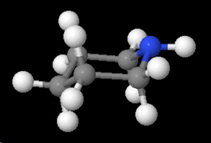
and the space-filling model.
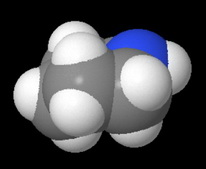
Morphine
The structure of morphine is
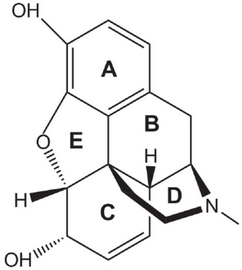
(From ResearchGate)
Ring D in its structure is a piperidine ring.
The methyl group on the nitrogen group should make morphine more basic than piperidine.
However, morphine is a weaker base, with
Morphine has a rigid pentacyclic ring structure, and the nitrogen atom is "buried" in the interior of the molecule, where it is less accessible to an attacking acid.
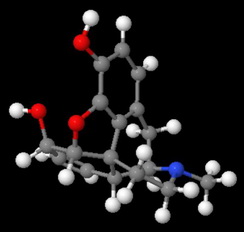
This becomes even clearer in a space-filling model of morphine.
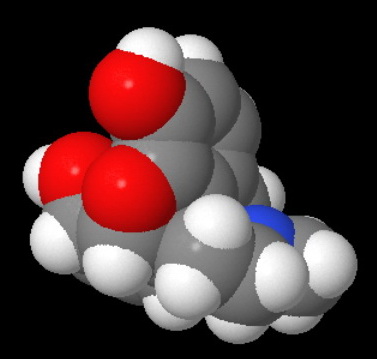
Attack by an acid is strongly hindered on one side by Ring A.
We find basicities by measuring the position of an equilibrium:
If access to the base is hindered, the position of equilibrium lies further to the left, and we say that the base is weaker.
Thus, morphine is a weaker base than piperidine.

