What is the product of reacting #"Br"_2# in methanol with methylenecyclohexane?
1 Answer
For determining the product in these type of questions, we have to look at the functional groups of the reactants. In this case, we have a
Now the
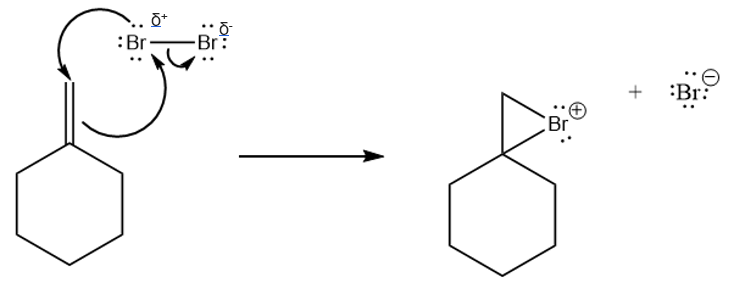 The Br will be added to the structure in a cyclic formation
The Br will be added to the structure in a cyclic formation
The other
Now we add
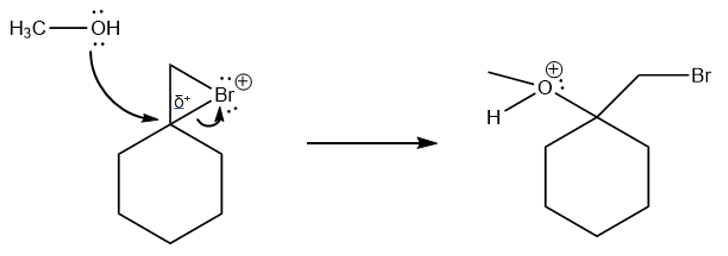 -
-
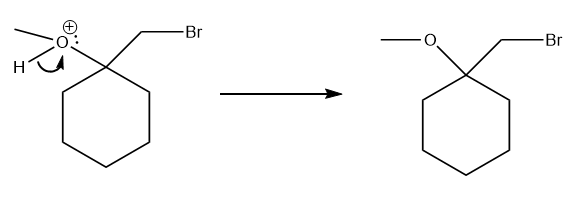 The electrons of the O atom can attack the partial positive carbon atom.
The electrons of the O atom can attack the partial positive carbon atom.
The carbon atom of the cyclic bromine group is partial positively charged, as indicated in the image above. This is created by hyperconjugation. This carbon atom is attached to 3 other carbon atoms (tertiary carbon atom), which stabilises a charge on the carbon atom more easily. Therefore the electrons from methanol will attack at that carbon atom, 'pushing away' the electrons from the bromine bond.
In the last step, the
And there you have your product!
