Question #43f8e
1 Answer
LeChatelier's principle => Stress is increase in
Explanation:
LeChatelier's Principle => If a stress is applied to a reaction, the reaction will shift away from the applied stress and establish a new equilibrium.
Three 'stress' factors affect stability of an equilibrium ...
1. Changes in concentration of reactants or products,
2. Temperature changes, and
3. Pressure-Volume effects (Boyle's Law)
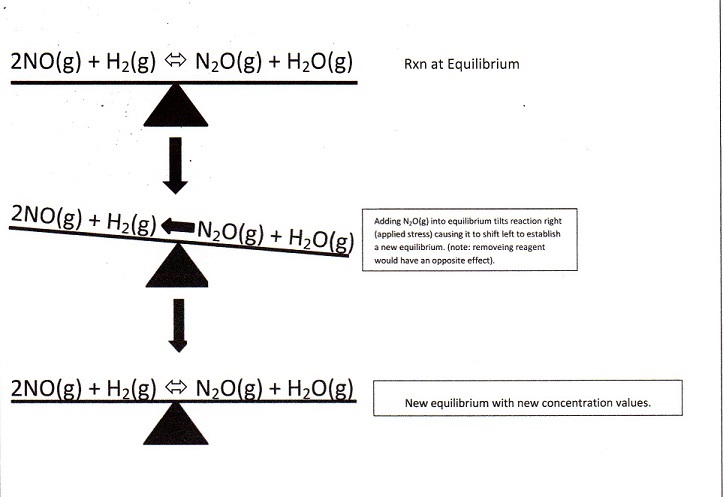
While considering concentration effects, it is useful to think of an equilibrium as being like a see-saw that is balanced. While at equilibrium, the see-saw is balanced and horizontal. However, if a reactant or product is added or subtracted, the balance will be disturbed and the reaction will no longer be in equilibrium. For the reaction to return to balance, the chemical process will occur to shift away from the applied stress. See the following figure =>