How many primary alcohols are possible given a formula of #C_5H_11OH#?
1 Answer
Apr 29, 2017
Well, I can count at least 4 such primary alcohols.........
Explanation:
And these are............:
And I just found this illustration, which I hope is right..........
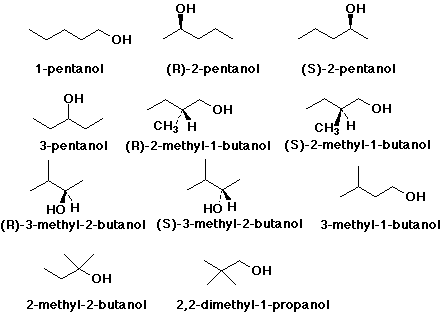
And this gives the four isomeric primary alcohols as required. Of course,

