What equation relates concentration to temperature t?
1 Answer
You are looking for the integrated rate law.
Explanation:
Your regular rate law is usually something of the form:
Rate
For a chemical reaction
This sort of rate law gives you an instantaneous rate of reaction at any given concentrations of
However, what happens when we want to know the rate at a given time? We can do this by using the integrated rate law, which is derived by integrating the aforementioned rate law with respect to time (derivation for this can be found in this video ).
The integrated rate law varies given the order of the reaction. This is summed up in the graphic below:
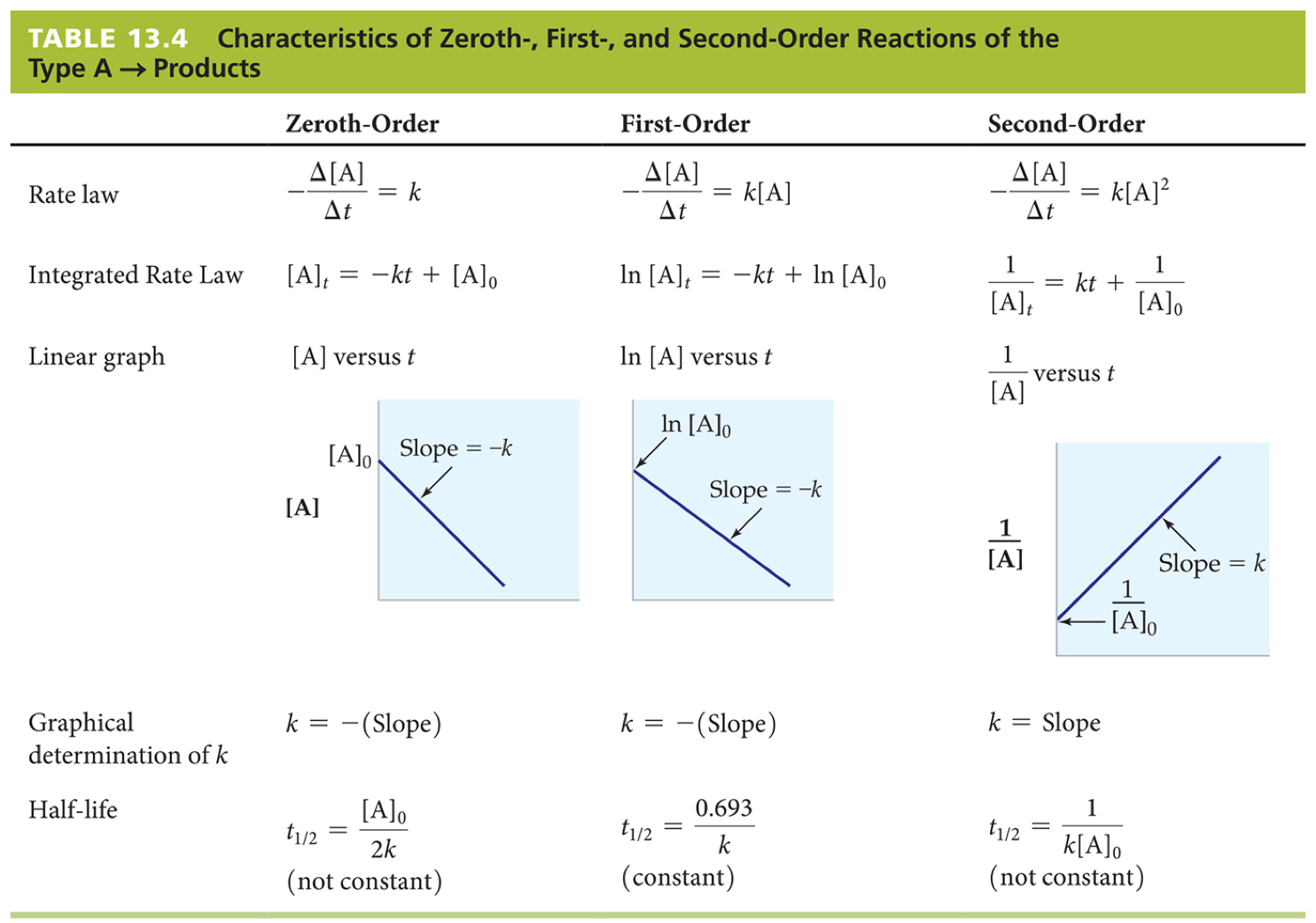 Chemistry, 7th Ed.
Chemistry, 7th Ed.
More information on the integrated rate law can be found in this video (same as the one linked above).
Hope that helps :)

