Question #b53c9
1 Answer
May 13, 2017
See below.
Explanation:
Various industrial processes produce gases that contain
In amine gas treating, these gases are passed through aqueous solutions of amines to remove the
The term lean amine refers to the amines used in these reactions.
Once the lean amine has absorbed all the sour gas constituents, it becomes rich amine, which can be collected in an absorber unit during the treating process.
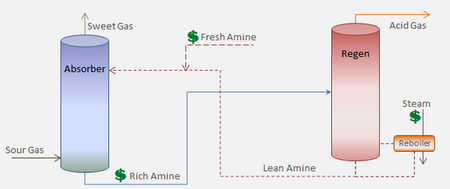
The reactions are

