Why is the melting point of hexafluorobenzene in a 1:1 mixture with benzene so much higher than the individual melting points of about #5^@ "C"#?
1 Answer
The high melting point is caused by π-π stacking of the aromatic rings.
Explanation:
In organic chemistry, π–π stacking refers to attractive interactions between the π clouds of aromatic rings.
There are various types of stacking.

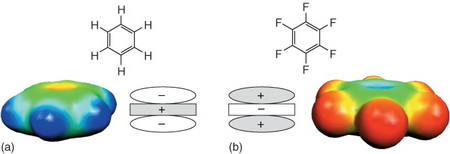
Neither benzene nor hexafluorobenzene has a dipole moment.
However, they have strong quadrupole moments, caused by the π clouds above and below the rings.
For example, in benzene, the π clouds are negatively charged and the plane of the ring is positively charged.

The situation is reversed in hexafluorobenzene, because the electronegative fluorine atoms withdraw electron density from the ring.
(From download.e-bookshelf.de)
You can see the charge distribution better in this image:

Both benzene and hexafluorobenzene are destabilized by sandwich stacking, because areas with the same charge are placed next to each other.

However, benzene and hexafluorobenzene are strongly stabilized by sandwich stacking, because areas with opposite charge are placed next to each other.
Theoretical calculations put the stabilization energy at about 20 kJ/mol.
That makes the attractions as strong as many hydrogen bonds and dipole-dipole interactions.
Thus, the strong intermolecular quadrupole attractions cause a 1:1 mixture of benzene and hexafluorobenzene to have a high melting point.

