How can we find the number of protons and electrons present in a neutral atom?
1 Answer
Grab a Periodic Table!
Explanation:
The number of protons present inside the nucleus of a given element is given by the atomic number, which is listed in the Periodic Table of Elements.
For example, carbon,
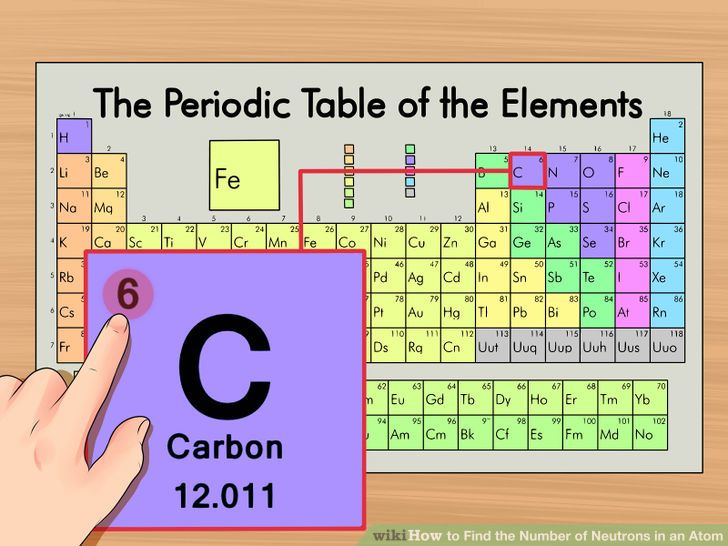
Now, a neutral atom will always have equal numbers of protons inside the nucleus and of electrons outside the nucleus.
#color(red)(ul(color(black)("no. of protons = no. of electrons "))) implies color(darkgreen)(ul(color(black)(" neutral atom")))#
This implies that for neutral atoms, the atomic number, which gives you the number of protons located inside the nucleus, will also give you the number of electrons that surround the nucleus.
In carbon's case, a neutral carbon atom has
So, to figure out the number of protons and electrons present in a neutral atom, find the element in the Periodic Table and look for its atomic number.

