Question #739ef
2 Answers
The question is perhaps asked backward: why do weaker interparticle forces cause things to be liquids while stronger interparticle forces cause them to be solid?
Explanation:
I have spoken of 'interparticle' rather than 'intermolecular' forces because not all solids and liquids are made of molecules.
Water, in solid and liquid phase, is made of molecules. The bonds between the molecules are hydrogen bonds. These are strong enough to hold the structure rigid when the temperature is low, leading to solid ice, but weaker allowing the molecules to move past each other when temperatures are higher and the water is in liquid phase. (weaker again in gas phase)
Solid metals have metallic bonding and are not made of molecules, but the forces are weaker in liquid phase than in solid phase. Ionic substances such as common salt (sodium chloride) show the same pattern.
It is the strength of the bonds that causes matter to be solid, and the relative weakness of the bounds that causes it to be liquid.
The kinetic energy of liquids is higher than the kinetic energy of solids and molecules in a liquid can therefore, overcome the energy barrier to moving around due to the intermolecular forces.
Explanation:
Molecules are always in motion. They can move around, rotate or vibrate. Each motion has its own energy with it, let's take for example translational energy for a molecule. The kinetic energy is then given by:
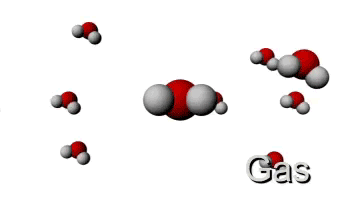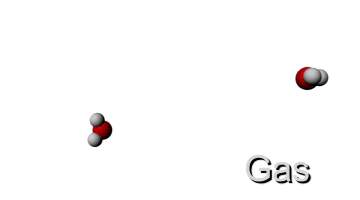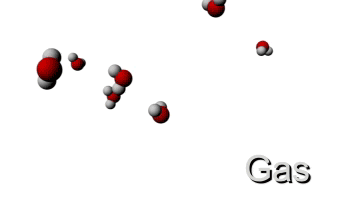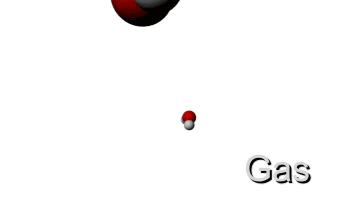
Now let's see what happens if we give the molecule more energy by increasing the temperature. This means that the kinetic energy will go up and since the mass stays the same, the velocity must increase too. Therefore the molecule will have a higher velocity when the temperature is increased. This is visualised below.
HIGH TEMPERATURE
 http://www.ergopedia.com/ergoweb/pedagogy_technology.php
http://www.ergopedia.com/ergoweb/pedagogy_technology.php
LOW TEMPERATURE
 i.imgur.com
i.imgur.com
(same source as above)
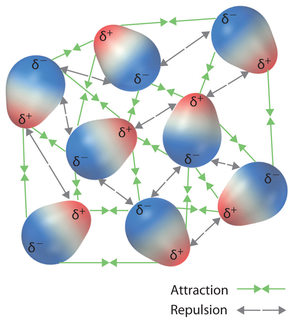
The interactions between the molecules are caused by the intermolecular forces or Van der Waals forces, of which I provided a list below briefly explaining some.
So we know now that there are forces between molecules and that these forces can attract or repel molecules and that the molecules are moving around.
Now if the intermolecular force isn't strong enough to keep the molecules together they can move around (higher velocity). So for example in a gas, the kinetic energy of the molecules is pretty high (they are moving fast) and the intermolecular forces aren't strong enough to keep them together.
For example, at
The different type of intermolecular interaction are listed below.
-
Dipole-dipole interaction
Two polar molecules (such asH-Cl ) are attracted to each other by dipole-dipole forces, for example,+ and- are attracted to each other. These differences in partial charges are created by the difference in electronegativity of the atoms. See picture below.
 https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/s15-02-intermolecular-forces.html
https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/s15-02-intermolecular-forces.html -
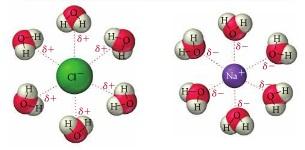
Ion-dipole force
This force also originates from the Coulomb force and is differs from the dipole-dipole interaction by having an ion (such asCl^- ) interaction with a dipole.
 classconnection.s3.amazonaws.com
classconnection.s3.amazonaws.com -
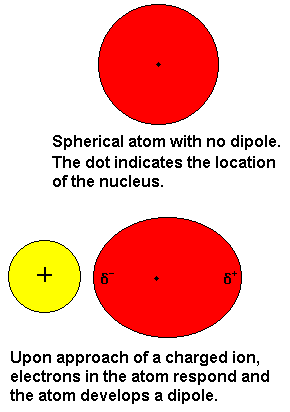
Ion-induced dipole force and dipole-induced dipole force.
The principle of these two interactions is the same. On its own, an atom does not have a charge, but when placed close to an ion or dipole, the electron cloud gets deformed and the atom will become an induced dipole.
 https://www.chem.purdue.edu/gchelp/liquids/inddip.html
https://www.chem.purdue.edu/gchelp/liquids/inddip.html -
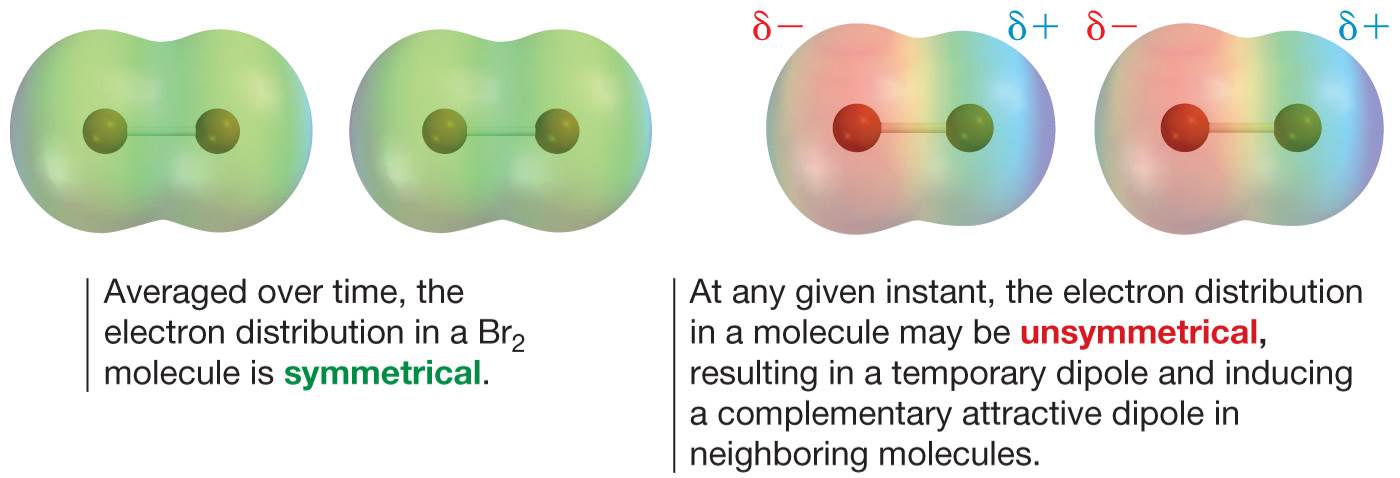
London dispersion force
This force is created by electron collection. When two alkane molecules approach each other, repulsion of the electrons in one molecule by those in the other results in the correlation of their movement. Correlated electron motion in the bonds of the other induces polarisation in the opposite direction, resulting in attraction between the molecules.
 https://sakai.ithaca.edu/access/content/user/jkleingardner/Principles%20HTML%20slides/ch10.html#/
https://sakai.ithaca.edu/access/content/user/jkleingardner/Principles%20HTML%20slides/ch10.html#/
