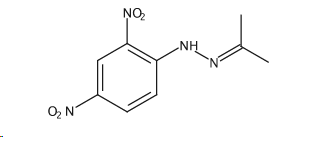
Help me annotate my IR spectrum of 2-Propanone, 2-(2,4-dinitrophenyl)hydrazone?
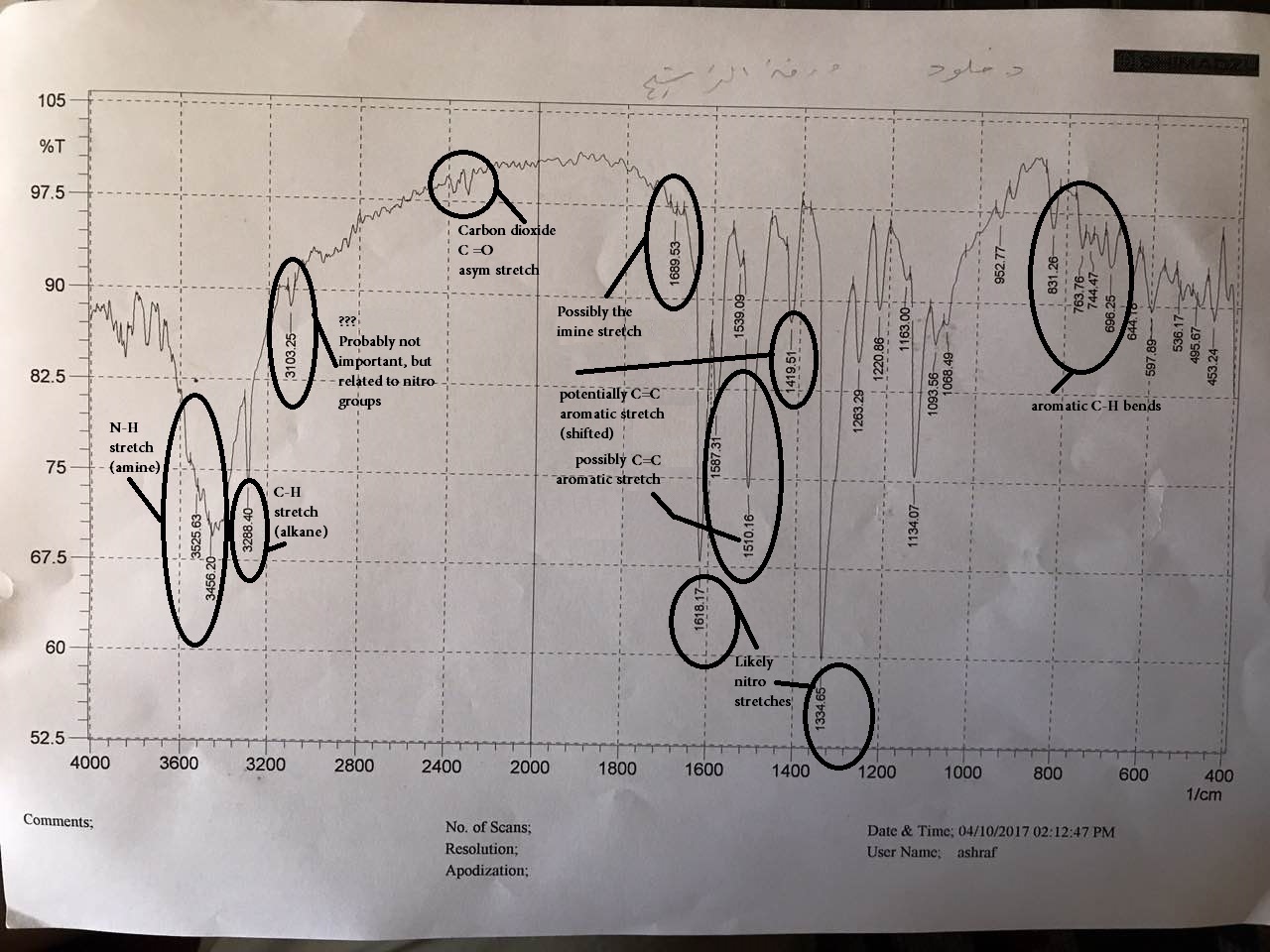
1 Answer
Based on this spectrum of nitrobenzene from SDBS (http://sdbs.db.aist.go.jp/), I don't think the
It may be the weak
(I am referring to the weak shoulder to the left of the two nitro stretches.)
If not, it may be the
(A weaker bond has a lower force constant and thus a lower fundamental stretching frequency.)
I searched your compound on http://scifinder.cas.org/, and landed on:

REFERENCE FREQUENCIES
From my text (Techniques in Organic Chemistry 3rd ed. by Mohrig), these are the relevant reference frequencies that I can find off-hand:
#bb("NO"_2)# stretches (#"1570 - 1490 cm"^(-1), "1390 - 1300 cm"^(-1)# , strong)- Aromatic
#"C"="C"# stretch (#"1620 - 1440 cm"^(-1)# , medium to weak),
#"C"-"H"# stretch (#"3100 - 3000 cm"^(-1)# , medium to weak),
#"C"-"H"# bend (#"900 - 680 cm"^(-1)"# , strong) - Amines
#"N"-"H"# stretch (#"3550 - 3250 cm"^(-1)# , medium;
#2^@# amine#=># one band) - Alkanes
#"C"-"H"# stretch (#"2990 - 2850 cm"^(-1)# , medium to strong)
Overall, here's what I came up with:
REFERENCE SPECTRA
To compare, here is a spectrum from the Bio-Rad/Sadtler IR Data Collection (Bio-Rad Laboratories, Philadelphia, PA), taken with a BIO-RAD DIGILAB FT-IR:
Since the amine frequencies weren't clear, another reference spectrum is from the Integrated Spectral Database System of Organic Compounds (National Institute of Advanced Industrial Science and Technology [Japan]), taken with a JASCO FT/IR-410:
Lastly, this website reports about

