Question #0f057
1 Answer
Analogue (B) has a greater amount of bioactive conformer
Explanation:
The bioactivity of a drug is mainly determined by the ability of the drug to bind with proteins which come on the way of the drug while it even reaches the receptors.
Reactive drugs easily bind with proteins or a macromolecule and cannot perform its bioactive role. To determine which analogue of drug is more bioactive you have to determine which drug is more stable than the other.
Galapentin is a drug which blocks voltage dependent calcium channels by binding to a receptor (a2

Features that determine the stability of two conformations are
Angle strain
Torsional strain
1,3 Diaxial strain
Van der Waals strain
Syn-pentane strain
Allylic strain
Ring strain
Bicyclic systems
If we look at both the analogue if you draw the chair conformer of both the analogues you would realize that the methyl group is in axial position in analogue A and equatorial in analogue B.
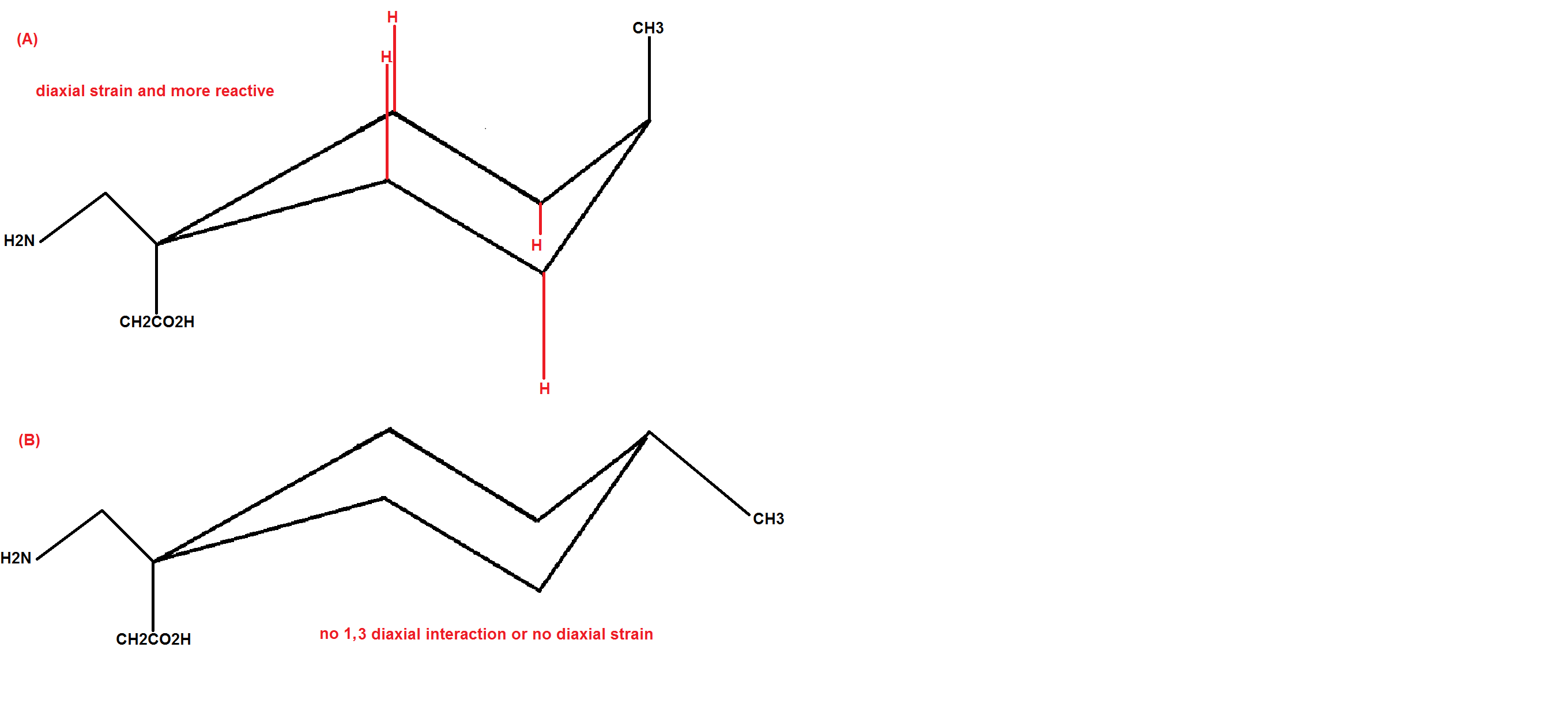
The

