Question #9db85
1 Answer
Explanation:
In order to be able to answer this question, you must know the enthalpy of fusion of water
#DeltaH_"fus" = "333.55 J g"^(-1)#
The enthalpy of fusion tells you the amount of energy needed in order to convert
In your case, you know that in order to convert
You can thus say that
#3 * 10^8 color(red)(cancel(color(black)("J"))) * "1 g ice"/(333.55color(red)(cancel(color(black)("J")))) = color(darkgreen)(ul(color(black)(9 * 10^5color(white)(.)"g ice")))#
The answer is rounded to one significant figure.
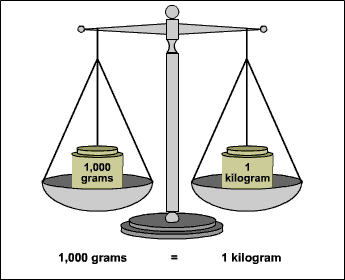
If you want, you can convert this to kilograms by using the fact that