How does water boil in a pot without a lid?
1 Answer
Vapor pressure builds up more easily in a closed container, but it can still increase in an open container.
Boiling in an open pot occurs once the rate of decrease in vapor pressure due to air dissolving into the water is insignificant compared to the rate of increase in vapor pressure due to the water heating up, AND the vapor pressure manages to match the atmospheric pressure.
Suppose we heat up a pot of solvent to boil it, open to the air.

We will have two competing processes as the solvent heats up:
- The particular solvent molecules with enough kinetic energy vaporize from the surface of the solution so that the vapor pressure above the solution increases.
- The air above the solution dissolves into the solvent to lower its vapor pressure by blocking the solvent particles from escaping the surface of the solution.
This is shown in this modification of Raoult's law:
#DeltaP_(vap) = -chi_"solute"P_(vap)^"*"# where
#chi_"solute"# is the mol fraction of any solute in an ideal solution, and#P_(vap)^"*"# is the vapor pressure of the pure solvent. Note that#DeltaP_(vap) < 0# , indicating a decrease is inevitable.
The solubility of gases in solvents (such as water) decreases with increasing temperature since faster particles have a larger escaping tendency. In other words, as the solvent is heated up, less air can be dissolved into the solvent.
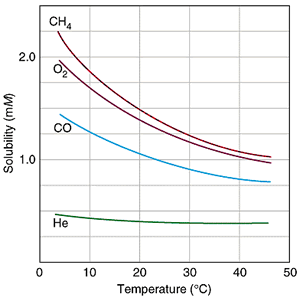
As a result, the decrease in vapor pressure due to air dissolving into the solvent becomes less significant as the solvent temperature increases.
(This is mathematically shown above, where a decrease in solubility [proportional to
Eventually, at hot enough temperatures, the rate of solvent vaporization (increase in vapor pressure) overcomes the rate of dissolving air in the solvent (decrease in vapor pressure), and the solvent (such as water) can reach its boiling point.

