Question #9a656
1 Answer
May 22, 2017
The name of the compound is 1-chloro-2-methylpentane.
Explanation:
Let's convert your condensed structure to a bond line structure.
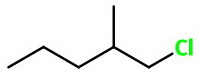
We see that the longest continuous chain of carbon atoms is the five-carbon chain going from left to right.
The base name of the compound is therefore pentane.
Next we number the carbon atoms, starting from the end nearest a substituent.
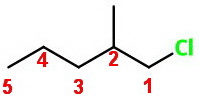
There is a chlorine atom on
We put the names of the substituents in alphabetical order in front of the base name.
The name of the compound is 1-chloro-2-methylpentane.

