Question #cc8dd
1 Answer
The correct answer is (a);
Explanation:
We're asked to find how the relative amounts of
To understand this aspect of chemical equilibrium, let's examine the effect of temperature change on a system at equilibrium, by looking at Le Châtelier's principle. We can start by forming general equations for endothermic and exothermic reactions, treating the accompanying heat as a chemical reagent:
If a reaction is endothermic, heat is treated as a reactant, as it is necessary to form the product(s):
Reactants + heat
#rightleftharpoons# productsIf a reaction is exothermic, heat is treated as a product, as it is released when the reaction occurs:
Reactants
#rightleftharpoons# products + heat
Le Châtelier's principle states that if the temperature of a system at equilibrium is increased, the system reacts as if a reactant (heat) was added to the system for an endothermic reaction, or as if a product (heat) was added to the system for an exothermic reaction.
Also according to Le Châtelier's principle, if the concentrations of one or more of the reactants is increased, the system will shift in the direction that consumes more reactants and forms more products (to the right).
And vice versa: if the concentrations of one or more of the products is increased, the system will shift in the direction that consumes more products and forms more reactants (to the left).
Thus, for a increase in temperature, the equilibrium will shift in the direction that consumes the added heat, and forms more of the other side.
And for a decrease in temperature, the equilibrium will shift in the direction that forms more heat, and thus shifts toward the side where heat is.
In the question, it's asking where the equilibrium will shift when heat is added (temperature increased) to an endothermic reaction. Heat is treated as a reactant for an endothermic reaction. We can now say the reaction will shift away from the heat; that is, away from the reactants side, and
To get a different perspective, here is a visualization of the effects of changing the temperature on both an endothermic and an exothermic reaction:
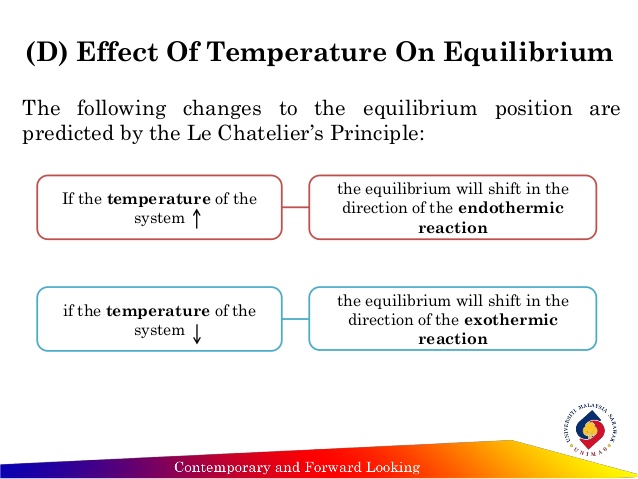
To summarize this visual, if the temperature of a system increases, regardless of whether it's endothermic or exothermic, equilibrium will shift in the direction of the endothermic reaction; i.e., toward the side that consumes more heat. If the temperature decreases, the equilibrium will shift in the direction of the exothermic reaction; i.e., toward the side that forms more heat.
I hope this helped clear any confusions you had! And I apologize for the long answer, I just wanted to make things as clear as I could:)
