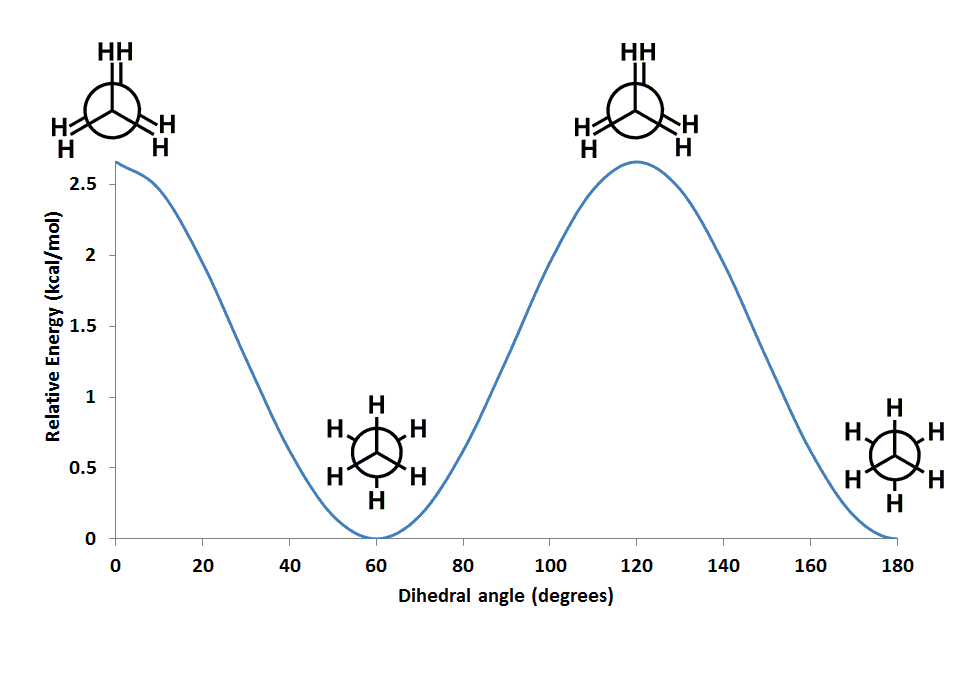
Which conformational isomer of ETHANE should be LEAST stable?
1 Answer
Consider the diagram.........
Explanation:
 chem.wisc.edu
chem.wisc.edu
We are looking down the
This is an example of conformational analysis........and your text will have a lot of examples of this sort of approach, especially with regard to carbocyclic 6-membered rings........
Note that when you try to interpret the relative energies of such conformations there is NOTHING childish or facile in using a model to inform your reasoning, and help you draw the conformation. In my own experience, I have seen a lot of good students FORGO the use of models in exams, and their marks have suffered accordingly.

