If light of wavelength "434 nm" irradiates a hydrogen atom, what energy level transition will it make? What series is this in the emission line spectrum?
1 Answer
Explanation:
All you have to do here is to use the Rydberg formula for a hydrogen atom
1/(lamda_"e") = R * (1/n_1^2 - 1/n_2^2)
Here
lamda_"e" is the wavelength of the emitted photon (in a vacuum)R is the Rydberg constant, equal to1.097 * 10^(7) "m"^(-1) n_1 represents the principal quantum number of the orbital that is lower in energyn_2 represents the principal quantum number of the orbital that is higher in energy
Notice that you need to have
So, convert the wavelength from nanometers to meters
434 color(red)(cancel(color(black)("nm"))) * "1 m"/(10^9color(red)(cancel(color(black)("nm")))) = 4.34 * 10^(-7) "m"
Rearrange the Rydberg formula to isolate the unknown variables
1/n_1^2 - 1/n_2^2 = 1/lamda_"e" * 1/R
Plug in your values to find
1/n_1^2 - 1/n_2^2 = 1/(4.34 * color(blue)(cancel(color(black)(10^(-7))))color(red)(cancel(color(black)("m"))) * 1.097 * color(blue)(cancel(color(black)(10^7)))color(red)(cancel(color(black)("m"^(-1)))))
1/n_1^2 - 1/n_2^2 = 0.21
This will be equivalent to
(n_2^2 - n_1^2)/(n_1 * n_2)^2 = 21/100
At this point, you have two equations with two unknowns, since
{(n_2^2 - n_1^2 = 21), (n_1 * n_2 = sqrt(100)) :}
Use the second equation to write
n_1 = 10/n_2" "color(darkorange)("(*)")
Plug this into the first equation to get
n_2^2 - (10/n_2)^2 = 21
n_2^2 - 100/n_2^2 = 21
This is equivalent to
n_2^4 - 100 = 21 * n_2^2
n_2^4 - 21n_2^2 - 100 = 0
If you take
x^2 - 21x - 100 = 0
Use the quadratic equation to get
x_(1,2) = ( -(-21) +- sqrt( (-21)^2 - 4 * 1 * (-100)))/(2 * 1)
x_(1,2) = (21 +- sqrt(841))/2
x_(1,2) = (21 +- 29)/2 implies {( x_1 = (21 - 29)/2 = -4), (x_2 = (21 + 29)/2 = 25) :}
Since we know that
n_2^2 = x
you can only use the positive solution here, so
n_2^2 = 25 implies n_2 = 5
According to equation
n_1 = 10/5 = 2
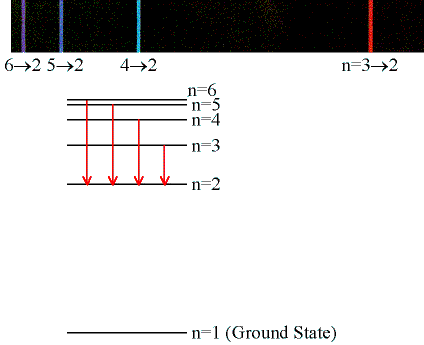
This means that your transition is taking place from
n=5 -> n=2
which is part of the Balmer series.
 http://www.daviddarling.info/encyclopedia/B/Balmer_series.html
http://www.daviddarling.info/encyclopedia/B/Balmer_series.html

