Can you tell from the chemical formula what the molecule looks like?
2 Answers
Yes. See the explantion.
Explanation:
Molecular substances that we become familiar with allows us to recognize a molecule from its chemical formula.
Several Examples
Sometimes yes, sometimes it's ambiguous.
- If the molecule has no more than one isomer, meaning that the chemical formula corresponds to only one molecule, then yes, you can.
- If the molecule has at least two isomers, then it's not obvious from the chemical formula what molecule it is.
Sometimes a chemical formula might even be written two different ways, and one way is clearer than another.
Examples:
#"CH"_3"COOH"# , or#"HC"_2"H"_3"O"_2# , are the same molecule, acetic acid:
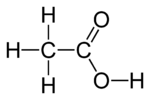
The first formula tells you the structure; it reads left to right correspondingly with the molecule.
The second formula is not useful to determine the structure, but it tells you that you have an acid since the first atom in the formula is an
#"H"# .
#"C"_2"H"_4"O"# has three isomers.It can be acetaldehyde,
#"CH"_3("C"="O")"H"# .
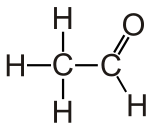
It can also be something crazy like ethenol,
#"H"_2"C"="CH"-"OH"# .
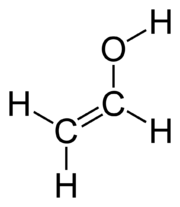
Then again, it could also be ethylene oxide, a three-membered ring with an oxygen atom for one of the ring atoms.
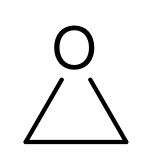
(where the four hydrogen atoms are implied, two on the bottom left and two on the bottom right.)
So you really can't tell what the molecule is... unless the formula is written suggestively.


