How do I do determine which half-reaction is at the cathode and anode in an electrolysis process??
#a)# Determine the half-reactions that occur at the anode and cathode for the electrolysis of #"NiBr"_2(l)# .
1 Answer
Summary:
- Determine what ions came from the compound.
- Write the reduction half-reactions that turn the neutral element into its ion by adding electron(s).
- Determine which half-reaction should be reversed based on which ion should be reduced or oxidized more easily, keeping in mind that the backwards (nonspontaneous) reaction to what you expect will be made to occur.
And remember, oxidation ALWAYS happens at the anode, and reduction ALWAYS happens at the cathode.
When you melt a solid into a liquid and then electrolyze it, notice the similarity to the word "electrolyte".
Electrolytes conduct electricity, and that is because they are ions that when placed into aqueous solution can carry and thus conduct electric charge with them.
Similarly, electrolysis of molten solids will produce their ions as PURE LIQUIDS and induce flow of electric current. Electric current among ions is due to the movement of electrons.
Therefore, we should begin by writing the preliminary half-reactions for the reduction of nonmetals and metal cations.
I'll do
Since you are looking at
So, the two reduction half-reactions so far are:
#"Br"_2(l) + 2e^(-) -> 2"Br"^(-)(l)#
#"Ni"^(2+)(l) + 2e^(-) -> "Ni"(s)#
Again, the reduction has it so that the atoms gain electrons.
Next, it is important to note that electrolysis forces a nonspontaneous process to occur by running electricity through it. This is going to correspond to the nonintuitive result, the result backwards from the spontaneous process.
Take this image and replace
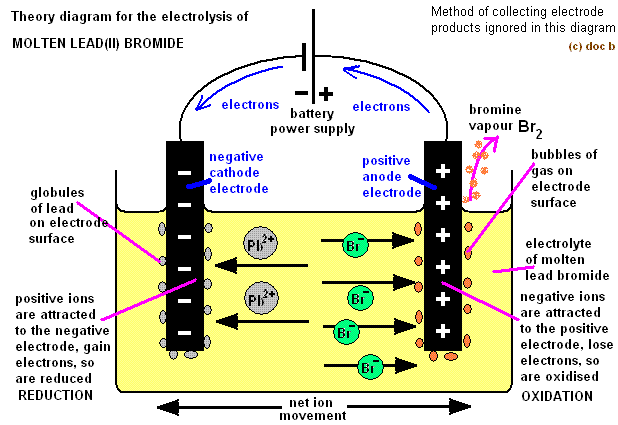
(The cathode is on the left, and the anode is on the right.)
Therefore, since we would normally expect halogens to have become reduced (to halides), the halide is actually oxidized (to the halogen), while the metal cation is reduced to the metal element.
#2"Br"^(-)(l) -> "Br"_2(l) + 2e^(-)# in the
#(+)# terminal, the anode
#"Ni"^(2+)(l) + 2e^(-) -> "Ni"(s)# in the
#(-)# terminal, the cathode
Overall, what we've found can be summarized:
- Determine what ions came from the compound.
- Write the reduction half-reactions that turn the neutral element into its ion by adding electron(s).
- Determine which half-reaction should be reversed based on which ion should be reduced or oxidized more easily, keeping in mind that the backwards (nonspontaneous) reaction to what you expect will be made to occur.

