Question #f3be3
1 Answer
Here's my explanation.
Explanation:
Bromothymol blue (HBtb) is a weak acid in which the molecular form and the ionized form have different colours.
The unionized form is yellow (below pH 6.0), while the ionized form is blue
(above pH 7.5).
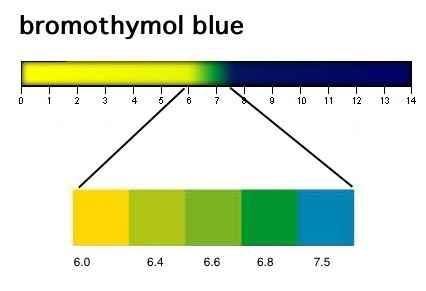
When you place
Le Châtelier's Principle states that, when a stress is applied to a system at equilibrium, the system will respond in such a way as to reduce the stress.
When you add
The system responds by producing more
At the same time, it produces more

