How is vapor pressure related to decreasing boiling point and the ease of boiling?
1 Answer
Jun 13, 2017
If the boiling point decreases, then it is easier to reach the boiling point. Thus, it is easier to boil.
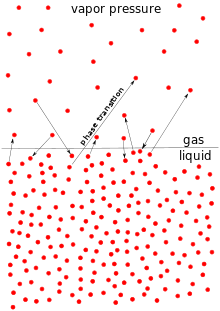
The vapor pressure is the pressure exerted by the vapor above the liquid form of the same substance. In order to boil, one needs the vapor pressure above the liquid to equal atmospheric pressure.
Therefore, if boiling is easier, then the amount of vapor above its liquid is higher, so it is easier to reach atmospheric pressure, and the vapor pressure is higher.
You can read more about this here:
https://socratic.org/questions/how-does-vapor-pressure-related-to-intermolecular-forces
In relation to the above link, it turns out that lower boiling point