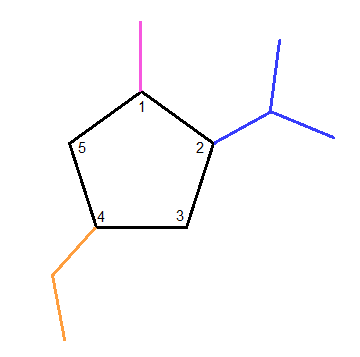
How do you draw this cyclic pentane? 4-ethyl-2-isopropyl-1-methylcyclopentane
1 Answer
See below
Explanation:
Let's break this down a bit.
First, your job is to identify the parent chain, which is the longest carbon chain.
That's easy as the last part of the structure's name gives it away:
Next, you have to identify the substituents. Well, knowing that cyclopentane is the parent chain, whatever else that we have left are treated as the substituents. Let's write them down.
#color(orange)"4-ethyl"# #color(blue)"2-isopropyl"# #color(magenta)"1-methyl"#
If we take our cyclopentane and first number all the carbons,
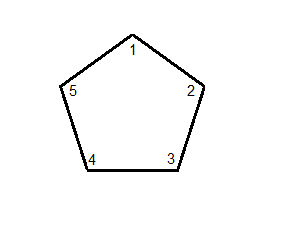
we can then, starting in numerical order, attach the substituents one-by-one.
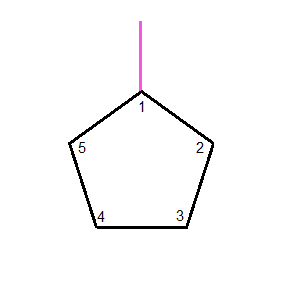
Looking at
Next, we take a look at
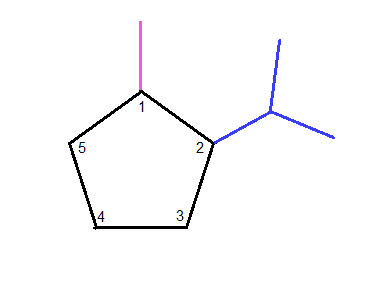
Lastly, looking at