How do you calculate the ionization energy of a hydrogen atom in its ground state?
1 Answer
Explanation:
!! VERY LONG ANSWER !!
Start by calculating the wavelength of the emission line that corresponds to an electron that undergoes a
This transition is part of the Lyman series and takes place in the ultraviolet part of the electromagnetic spectrum.
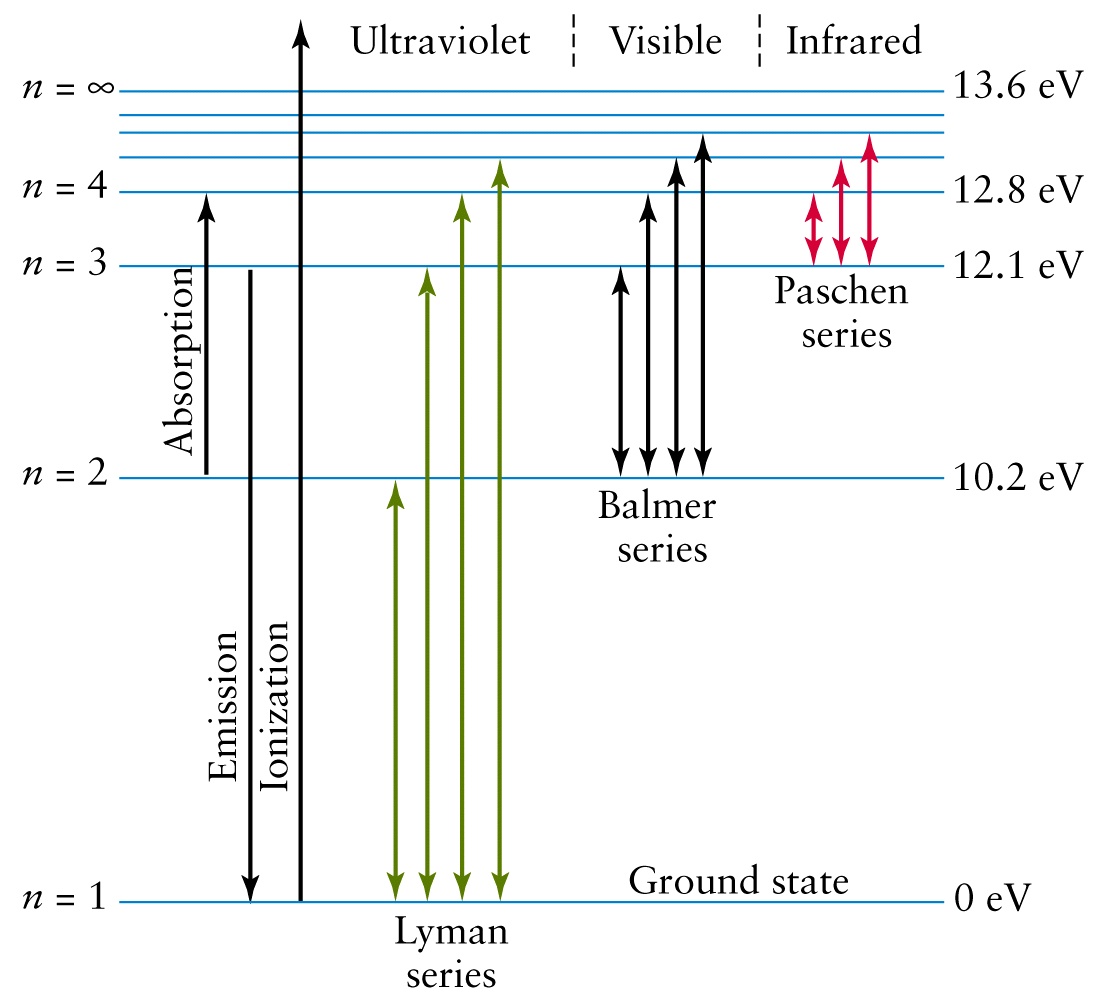 http://www.physast.uga.edu/~rls/1010/ch5/ovhd.html
http://www.physast.uga.edu/~rls/1010/ch5/ovhd.html
Your tool of choice here will be the Rydberg equation for the hydrogen atom, which looks like this
#1/(lamda_"e") = R * (1/n_1^2 - 1/n_2^2)#
Here
#lamda_"e"# is the wavelength of the emitted photon (in a vacuum)#R# is the Rydberg constant, equal to#1.097 * 10^(7)# #"m"^(-1)# #n_1# represents the principal quantum number of the orbital that is lower in energy#n_2# represents the principal quantum number of the orbital that is higher in energy
In your case, you have
#{(n_1 = 1), (n_2 = oo) :}#
Now, you know that as the value of
#1/n_2^2 -> 0#
This implies that the Rydberg equation will take the form
#1/(lamda) = R * (1/n_1^2 - 0)#
#1/(lamda) = R * 1/n_1^2#
which, in your case, will get you
#1/(lamda) = R * 1/1^2#
#1/(lamda) = R#
Rearrange to solve for the wavelength
#lamda = 1/R#
Plug in the value you have for
#lamda = 1/(1.097 * 10^(7)color(white)(.)"m") = 9.116 * 10^(-8)# #"m"#
Now, in order to find the energy that corresponds to this transition, calculate the frequency,
#color(blue)(ul(color(black)(nu * lamda = c)))#
Here
#nu# is the frequency of the photon#c# is the speed of light in a vacuum, usually given as#3 * 10^8# #"m s"^(-1)#
Rearrange to solve for the frequency and plug in your value to find
#nu * lamda = c implies nu = c/(lamda)#
#nu = (3 * 10^(8) color(red)(cancel(color(black)("m"))) "s"^(-1))/(9.116 * 10^(-8)color(red)(cancel(color(black)("m")))) = 3.291 * 10^(15)# #"s"^(-1)#
Finally, the energy of this photon is directly proportional to its frequency as described by the Planck - Einstein relation
#color(blue)(ul(color(black)(E = h * nu)))#
Here
#E# is the energy of the photon#h# is Planck's constant, equal to#6.626 * 10^(-34)"J s"#
Plug in your value to find
#E = 6.626 * 10^(-34)color(white)(.)"J" color(red)(cancel(color(black)("s"))) * 3.291 * 10^(15) color(red)(cancel(color(black)("s"^(-1))))#
#E = 2.181 * 10^(-18)# #"J"#
This means that in order to remove the electron from the ground state of a hydrogen atom in the gaseous state and create a hydrogen ion, you need to supply
This means that for
#"H"_ ((g)) + 2.181 * 10^(-18)color(white)(.)"J" -> "H"_ ((g))^(+) + "e"^(-)#
Now, the ionization energy of hydrogen represents the energy required to remove
To convert the energy to kilojoules per mole, use the fact that
You will end up with
#6.022 * 10^(23) color(red)(cancel(color(black)("photons")))/"1 mole photons" * (2.181 * 10^(-18)color(white)(.)color(red)(cancel(color(black)("J"))))/(1color(red)(cancel(color(black)("photon")))) * "1 kJ"/(10^3color(red)(cancel(color(black)("J"))))#
# = color(darkgreen)(ul(color(black)("1313 kJ mol"^(-1))))#
You can thus say that for
#"H"_ ((g)) + "1313 kJ" -> "H"_((g))^(+) + "e"^(-)#
The cited value for the ionization energy of hydrogen is actually
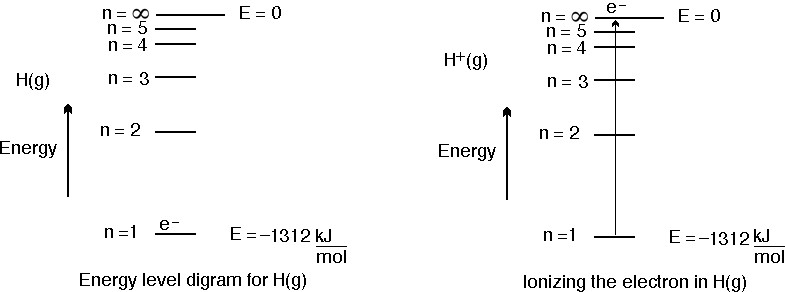 http://genchem1.chem.okstate.edu/BDA/BCE22.php
http://genchem1.chem.okstate.edu/BDA/BCE22.php
My guess would be that the difference between the two results was caused by the value I used for Avogadro's constant and by rounding.
#6.02 * 10^(23) -> "1312 kJ mol"^(-1)" vs "6.022 * 10^(23) -> "1313 kJ mol"^(-1)#

