Question #9fbfb
1 Answer
Explanation:
The trick here is to realize that sulfur exists as an octatomic molecule under standard conditions.
In other words, sulfur exists as molecules that contain
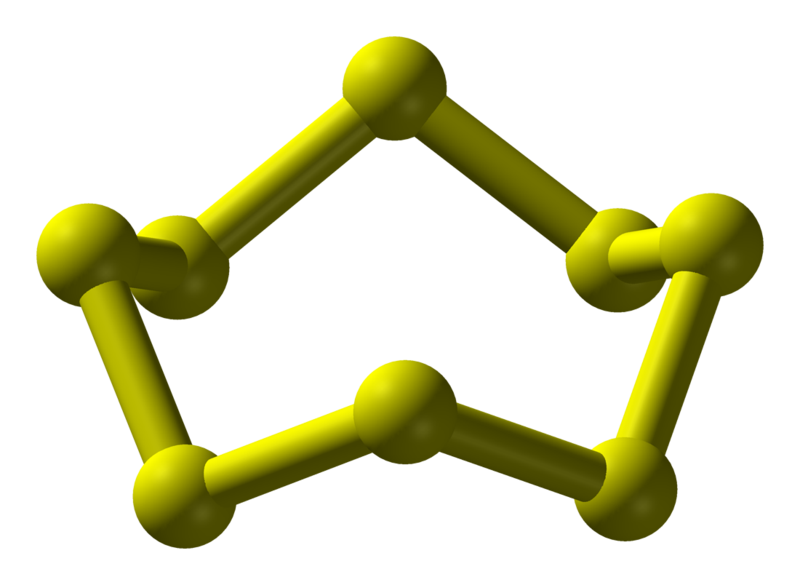
This implies that
#8 * overbrace("32.065 g mol"^(-1))^(color(blue)("the molar mass of elemental sulfur")) = "256.52 g mol"^(-1)#
You can thus say that
#3.2 color(red)(cancel(color(black)("g"))) * "1 mole S"_8/(256.52color(red)(cancel(color(black)("g")))) = "0.012475 moles S"_8#
The number of octatomic sulfur molecules present in this sample will be equal to
#0.012475 color(red)(cancel(color(black)("moles S"_8))) * overbrace((6.022 * 10^(23)color(white)(.)"molecules S"_8)/(1color(red)(cancel(color(black)("mole S"_8)))))^(color(blue)("Avogadro's constant")) = 7.51 * 10^(21)# #"molecules S"_8#
Since each molecule of octatomic sulfur contains
#7.51 * 10^(21) color(red)(cancel(color(black)("molecules S"_8))) * "8 atoms S"/(1color(red)(cancel(color(black)("molecule S"_8)))) = color(darkgreen)(ul(color(black)(6.0 * 10^(22)color(white)(.)"atoms S")))#
The answer is rounded to two sig figs, the number of sig figs you have for the mass of the sample.

