Question #66e91
1 Answer
Surfactants.
Explanation:
Surfactants are compounds that "lower the surface tension (or interfacial tension) between two liquids, between a gas and a liquid, or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants." (Wikipedia)
You can explain the mechanism of soap using the principle of like dissolve like. For example, ethanol (
Surfactant molecules in soap are usually amphiphile, meaning that they exhibit both polar and nonpolar characteristics. They help to break up chunks of insoluble materials which would otherwise be insoluble in water.
For example, Sodium lauryl sulfate (
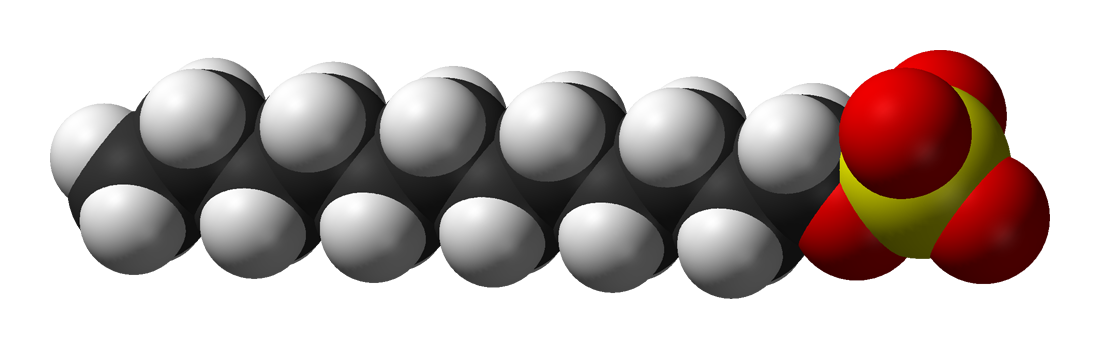
A
Sources:
Wikipedia contributors. "Surfactant." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 29 Dec. 2017. Web. 15 Jan. 2018.
Wikipedia contributors. "Petroleum ether." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 1 Aug. 2017. Web. 15 Jan. 2018.
Woodford, Chris. "How Do Detergents And Soaps Work?." Explain That Stuff, 2017, http://www.explainthatstuff.com/detergents.html.

