Question #50bb0
2 Answers
30 grams of NaOH.
Explanation:
Generally, the definition of % concentration for acids and bases is grams per 100 grams.
In this example, a 30% solution can be written as 30% wt./wt., which means that there are 30 g of NaOH in 100 g of final solution.
So you need to weigh out 30 g of NaOH and dissolve it in enough water to get a total mass of 100 g.
Explanation:
I'll assume that you mean that the desired solution is
If the solution (which I take to be only water and aqueous sodium hydroxide) were to have a mass of
and the remaining
Further information:
The solubility of sodium hydroxide in water is seen by its solubility curve:
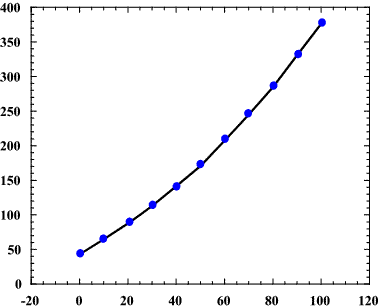 http://chemicals.etacude.com
http://chemicals.etacude.com
where the
Since there are
that dissolve, just barely above the
We can conclude that the solubility of sodium hydroxide in
Therefore, as long as the solution temperature is above
However, if the solution temperature is lower than

