What are directing groups?
1 Answer
Jun 30, 2017
They are bonded substituents that influence incoming electrophiles to yield towards the position ortho (1,2) or para (1,4) to the substituent.
The para position is favored if the incoming electrophile is large, but both kinds of products (ortho/para substitution) are usually formed in some ratio.
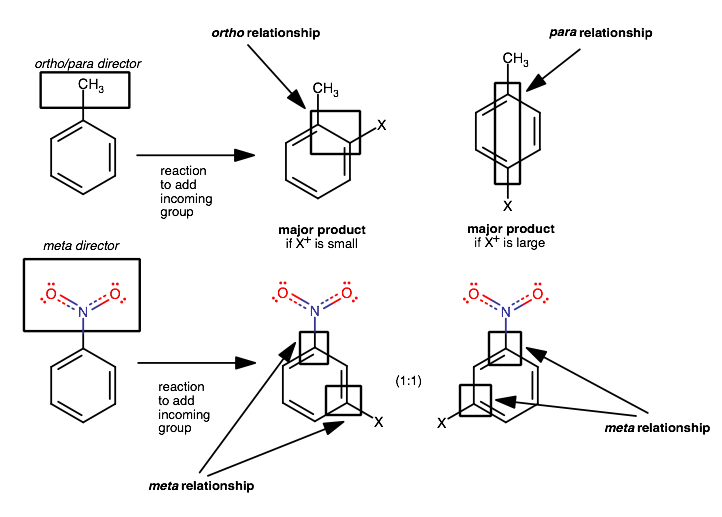
Ortho/para directors activate the aromatic ring for electrophilic aromatic substitution, directing the incoming electrophiles towards the carbon 1,2 or 1,4 to the directing group.
These are common kinds of ortho/para directing groups:
- electron-donating/releasing, e.g. alkyl, hydroxyl (
#-"OH"# ), alkoxy (#-"OR"# ), ammine (#-"NR"_2# ) - halides, e.g.
#-"F"# ,#-"Cl"# ,#-"Br"# ,#-"I"# , etc.
Meta directors, on other hand, evidently direct to the position 2 away from the meta director by deactivating the ring at the ortho and para positions.
These are common kinds of meta directing groups:
- electron-withdrawing (high EN atoms), e.g. trihalomethyl (
#"CCl"_3# ,#"CBr"_3# ,#"CF"_3# , etc.) - electron-withdrawing (and electron-dense), e.g. carbonyl (
#-stackrel("O")stackrel(||)("C")-"R"# , where#R# could be alkyl,#-"Cl"# ,#-"OH"# ,#-"OR"# , etc.), nitro (#-"NO"_2# ), nitrosyl (#-"NO"# ), cyano/nitrile (#-"CN"# ), hydroxysulfonyl (#-"SO"_3"H"# ), etc. - charged, e.g. protonated ammine (
#"NR"_3^(+)# )

