Question #0fc8e
1 Answer
Jul 1, 2017
Explanation:
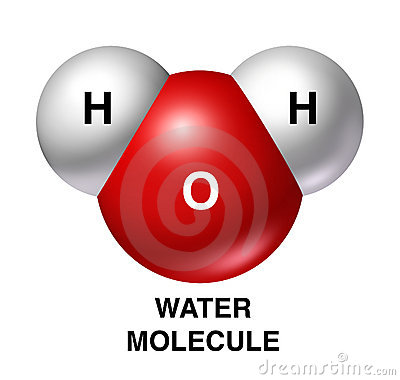
As its chemical formula suggests, a molecule of water contains
- two atoms of hydrogen,
#2 xx "H"# - one atom of oxygen,
#1 xx "O"#
This means that a single molecule of water contains a total of
#"no. of atoms in H"_2"O" = "2 atoms of H + 1 atom of O"#
#"no. of atoms in H"_2"O" = "3 atoms"#
So remember, we use subscripts to denote the number of atoms of each element present in a given compound. Keep in mind that we do not add a subscript of
So in this case, two atoms of hydrogen are shown by adding a subscript of
So instead of
#"H"_2"O"_1#
we have
#"H"_2"O"#

