Question #670ca
1 Answer
Explanation:
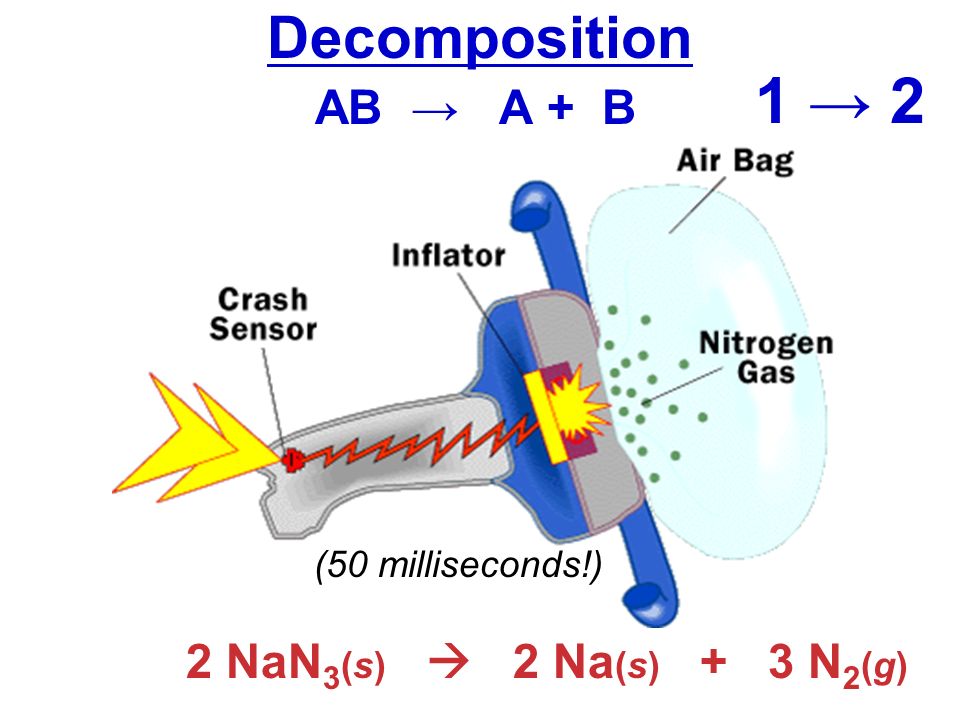
The decomposition of sodium azide to sodium metal and nitrogen gas can be described by the following balanced chemical equation
#color(blue)(2)"NaN"_ (3(s)) stackrel(color(white)(acolor(red)(Delta)aaa))(->) 2"Na"_ ((s)) + color(green)(3)"N"_ (2(g)) uarr#
Notice that for every
This means that your reaction will produce
#1 color(red)(cancel(color(black)("mole NaN"_3))) * (color(green)(3)color(white)(.)"moles N"_2)/(color(blue)(2)color(red)(cancel(color(black)("mole NaN"_3)))) = "1.5 moles N"_2#
Keep in mind that the answer must be rounded to one significant figure because that's the number of sig figs you have for your data.
This means that you have
#color(darkgreen)(ul(color(black)("moles of N"_2 = "2 moles")))#
As a fun fact, it's worth mentioning that this reaction is used to inflate airbags in cars.