How is #"n-hexane"# represented?
1 Answer
Jul 7, 2017
Explanation:
And a typical representation is....
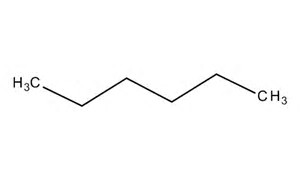
The carbon-carbon bonds are in the plane of the page, and each chain carbon has 2 hydrogens projecting inwards and outwards with respect to the page........
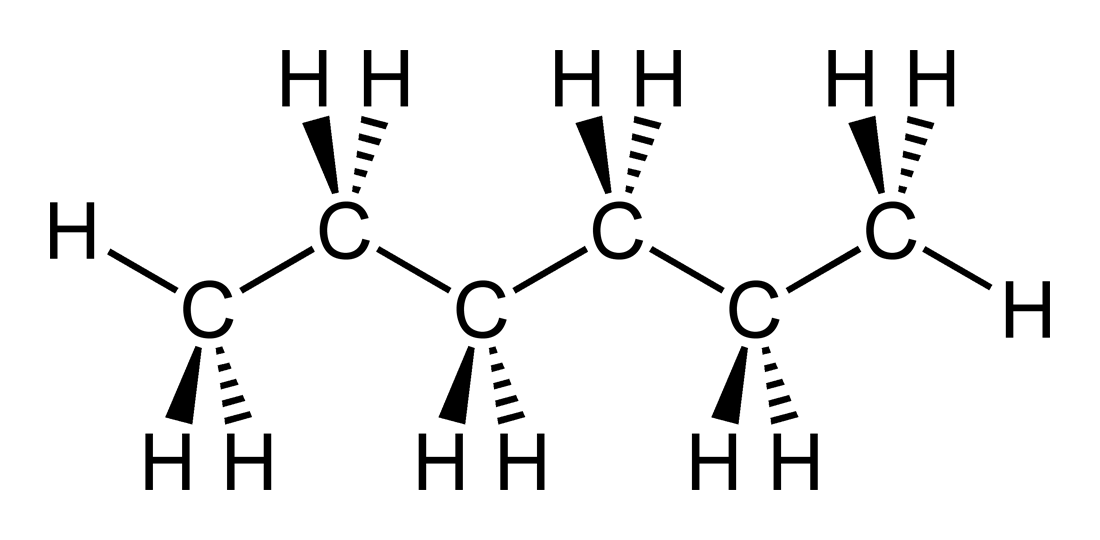
Of course, the molecule flips and flops about in solution, but when we represent it we assume the MAXIMUM SYMMETRY POSSIBLE (if it does not adopt this symmetry we find out pdq!). How many signals would appear in its

