How does #"ionization energy"# evolve in terms of the Periodic Table?
1 Answer
Well, ionization energy INCREASES across a Period.......
Explanation:
Well, ionization energy INCREASES across a Period, a ROW of the Periodic Table, from left to right as we face the Table.
And ionization energy DECREASES down a Group.
Why so? Well, there is a contest between (i) nuclear charge,
And thus we can account for measurable ionization energies, i.e. the energy associated with the following equation....
......on the basis of periodicity. Elements to the right of the Periodic Table (as we face it!) should have higher ionization enthalpies. As chemists, as physical scientists, we should interrogate the data.
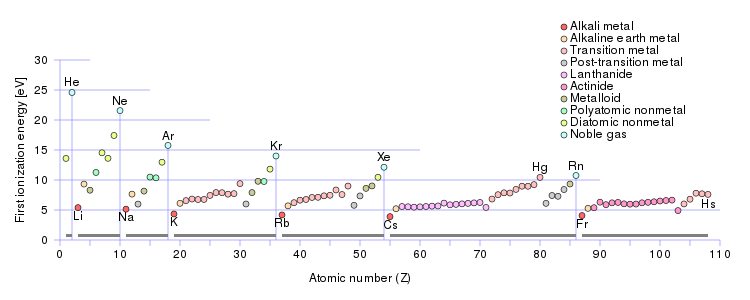
This is listed in
And after all that we gots..............
But that's round the wrong way in the order of INCREASING first ionization energy. And so......
Clearly, this follows

