Question #db655
1 Answer
Explanation:
I believe the answer you are looking for would be an isotope. An isotope is:
"each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties; in particular, a radioactive form of an element."
(credits to my computers Apple dictionary).
For instance:
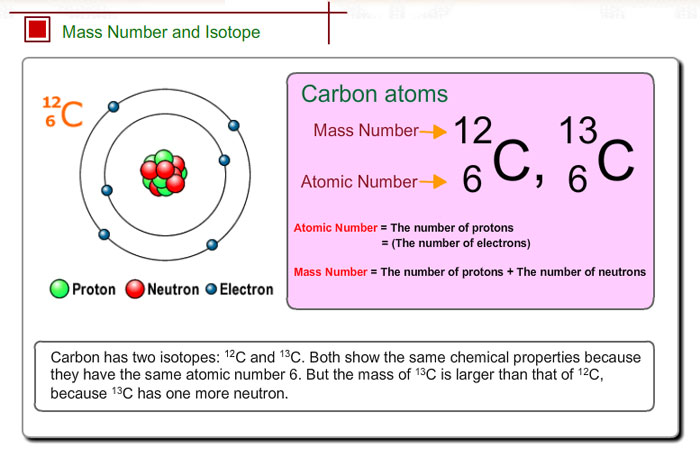
As you can see, these are isotopes of the element Carbon
Carbon-12 has:
However, Carbon-13 has:
Therefore, Carbon-13 has more mass than Carbon-12.
Isotopes really explain themselves. If you are given Carbon-14 for instance, then you can write the isotope with the given information:
Mass Number:
Atomic Number:
Therefore the isotope can be labeled as so:

