Question #65232
1 Answer
Explanation:
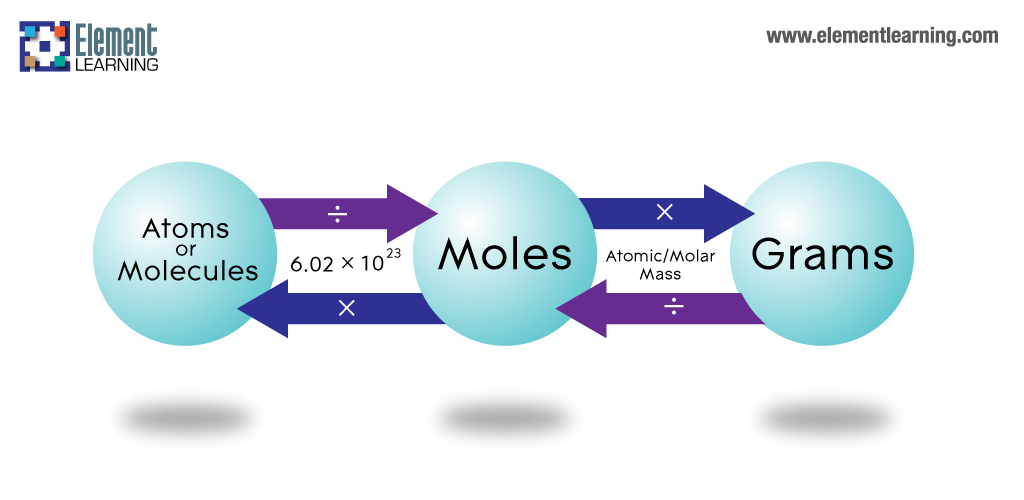
In order to be able to convert between atoms and grams, you need to first go through moles.
You can convert the number of atoms of magnesium to moles of magnesium by using the fact that, by definition, you need to have
In your case, the sample will contain
#2.01 * 10^10 color(red)(cancel(color(black)("atoms Mg"))) * overbrace("1 mole Mg"/(6.022 * 10^(23) color(red)(cancel(color(black)("atoms Mg")))))^(color(blue)("Avogadro's constant")) = 3.338 * 10^(-14)# #"moles Mg"#
Finally, to convert the number of moles of magnesium to grams, you must use the molar mass of the element, i.e. the mass of exactly
You will have
#3.338 * 10^(-14)color(red)(cancel(color(black)("moles Mg"))) * overbrace("24.305 g"/(1color(red)(cancel(color(black)("mole Mg")))))^(color(blue)("the molar mass of Mg")) = color(darkgreen)(ul(color(black)(8.11 * 10^(-13)color(white)(.)"g")))#
The answer is rounded to three sig figs, the number of sig figs you have for the number of atoms of magnesium present in the sample.