How do I identify #R# and #S# stereoisomers??? When is a carbon compound chiral?
1 Answer
You could have given us a bit more to go on.........
Explanation:
It is relatively straightforward to assign chirality to carbon compounds. Of course we have to have a depiction of the molecule.........and we ain't got one here. I can give you a few tips.
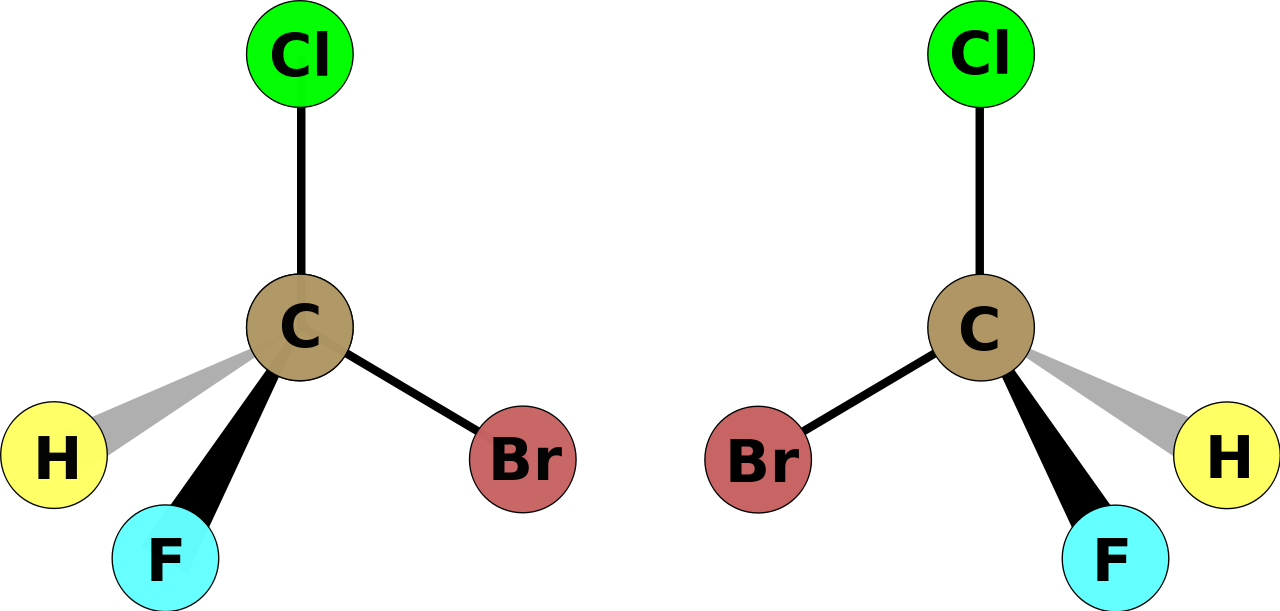
ANY carbon centre in a tetrahedral array that has four different substituents can generate a pair of optical isomers based on the geometry of its substituents. The one on the left (as we face it) is the
How? Well for each depicted stereoisomer, the substituent of least priority, the hydrogen, projects INTO the page.
Given such a stereoisomer, the interchange of ANY two substituents at one optical centre results in the enantiomer. Interchange again, (and it need not be the original two substituents) and you get the mirror-image of a mirror-image, i.e. the enantiomer of an enantiomer, that is the ORIGINAL isomer.
The big mistake that students tend to make with these problems
Of course you need to practise how to represent such models on the printed page UNAMBIGUOUSLY. Good luck.

