How would I know the enthalpy of formation of barium carbonate on an exam?
1 Answer
Aug 1, 2017
Well, then you'll have to identify the substance's chemical formula, which is fair play on any exam... You must also correctly identify the phase as seen in the chemical reaction that you are asked to examine.
A typical table of enthalpies of formation is:
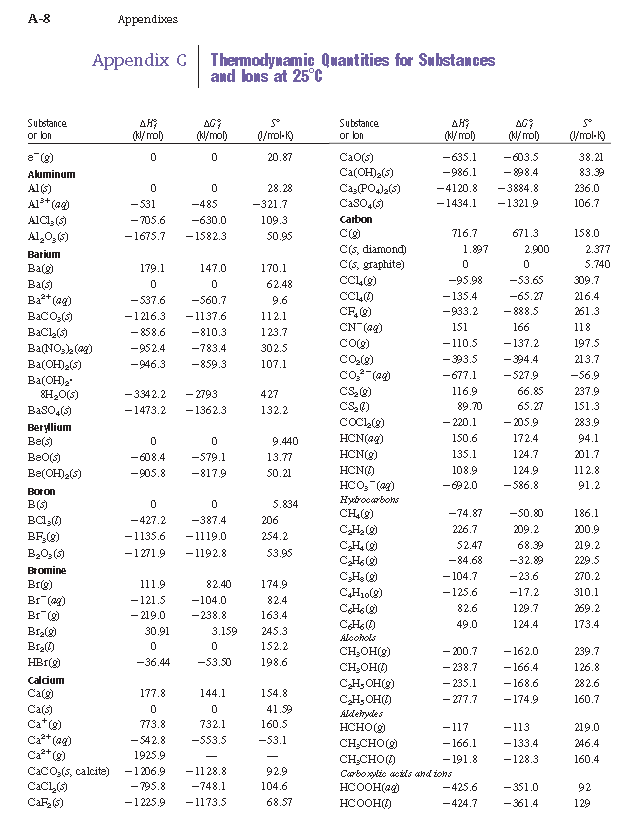
And you can see that there is no such chemical name listed for barium carbonate, but the chemical formula is somewhere on this page.

