What will be the uncertainty in momentum if we know the position certainly?
1 Answer
It would be infinite... which is clearly greater than
Heisenberg's Uncertainty Principle commonly applies to electrons, such that...
DeltaxDeltap >= h//4pi where
Deltaq is the uncertainty inq ,x is position, andp is momentum.h is Planck's constant.
The inequality requires that if one uncertainty gets too low, the other uncertainty must increase until the inequality is satisfied.
Thus, if
In fact, although we never have absolute certainty about an electron's position, we usually are more certain about the position of the electron rather than its momentum. The physical proof of that is:
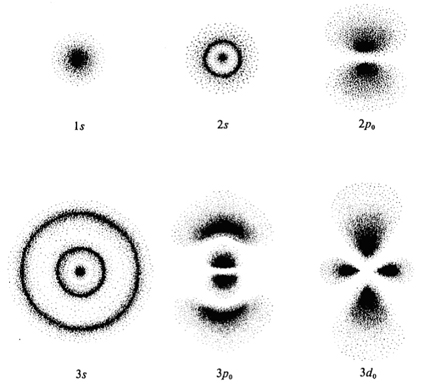 http://www.forgottenplanet.com/
http://www.forgottenplanet.com/
As the wave function (the solution to the Schrodinger equation) must be bounded, that generates what we regard as orbital probability densities, seen above.

