Question #10bcb
1 Answer
Here's how I would explain it.
Explanation:
Cyclic forms of glucose
There are actually two cyclic forms of glucose.
They are in equilibrium with the open-chain form.
They are stable cyclohexane-like rings in which all the bulky substituents are in equatorial positions (except the α-form, in which the
They are so much more stable than the open-chain form that the latter is only a minor component of the equilibrium mixture.

Epimerization of glucose
Galactose and mannose are epimers of glucose — they are optical isomers that differ in configuration at only one chiral centre.

Glucose and mannose can be converted into each other through an intermediate enediol.
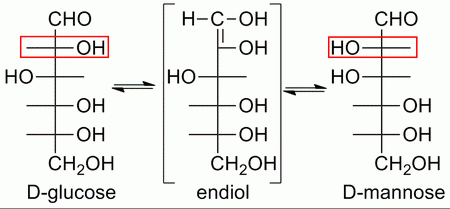
(From Wikimedia Commons)
However, the reaction is catalyzed by base; it is extremely slow in neutral solution.
The reaction depends on the adjacent carbonyl group for the epimerization at
Thus, galactose is not formed in this process.

