For a pair of enantiomers, which of the following properties are true? #a." The physical properties of enantiomers are different."# #b." Enantiomers have identical chemistry in all circumstances."# #c." Enantiomers have the same physical properties."#
1 Answer
I would choose
Explanation:
Enantiomers have the same physical and chemical properties in ALL ACHIRAL environments. Thus melting points, boiling points, are IDENTICAL. And certainly atom connectivities are the same (if they were not then they would not be
On the other hand enantiomers have DIFFERENT three-dimensional orientation; one is the mirror-image of the other.
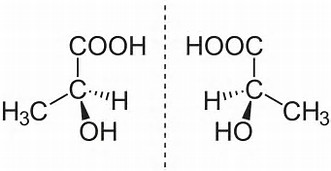
Note as a practical tip, given the ONE enantiomer, interchange ANY 2 substituents, and you generate the mirror image. Interchange again, (and it clearly does not have to be the original two substituents), and you get the enantiomer of an enantiomer, i.e. the ORIGINAL stereoisomer.
You should try this out with simple models, and also try out representing the three-dimensional structure on the printed page. It's not straightforward (at least I have never found it so), and practice will help develop your understanding, and your ability to show the examiner that you know how many beans make five.

